Flying Blind: unveiling the patchy landscape of global R&D for endometriosis, polycystic ovary syndrome and uterine fibroids
By Impact Global Health 10 September 2025
Common yet ignored: the neglect of gynaecological conditions in medical research
Endometriosis, polycystic ovary syndrome (PCOS) and uterine fibroids are common gynaecological conditions that affect the health and wellbeing of millions of women1 worldwide. Despite their high prevalence and burden, medical knowledge and therapeutic options are limited, because of a historical neglect of women’s health conditions.
Currently, the pathophysiology of these three conditions is poorly understood, although various pathways are implicated (e.g. endocrine, inflammatory, etc). As a result, clinical management relies on repurposed medicines for symptoms only, and there are no cures available aside from fertility impacting surgery, such as hysterectomy for uterine fibroids.
Diagnostics are also limited. Uterine fibroids are detected by ultrasound, while diagnosing PCOS relies on identifying a cluster of symptoms – often subjectively and using inconsistent thresholds – through clinical examination, ultrasound and endocrine biomarkers, many of which are inaccessible in low-resource settings. For endometriosis, the gold standard, and until recently, only available diagnostic, is laparoscopic examination. This is invasive and not always accessible, leading to severe diagnostic delays – estimated to be between 4 to 11 years – and a massive underestimation of disease prevalence.
Despite the high prevalence and burden associated with these common gynaecological conditions, the research and development landscape is underdeveloped and underfunded, leaving women with few diagnostic and therapeutic options to address or manage symptoms that seriously affect their quality of life. While endometriosis R&D has benefited from recent momentum, enormous gaps in knowledge persist – as they do across all three conditions – requiring greater and more ambitious investment to drive progress.
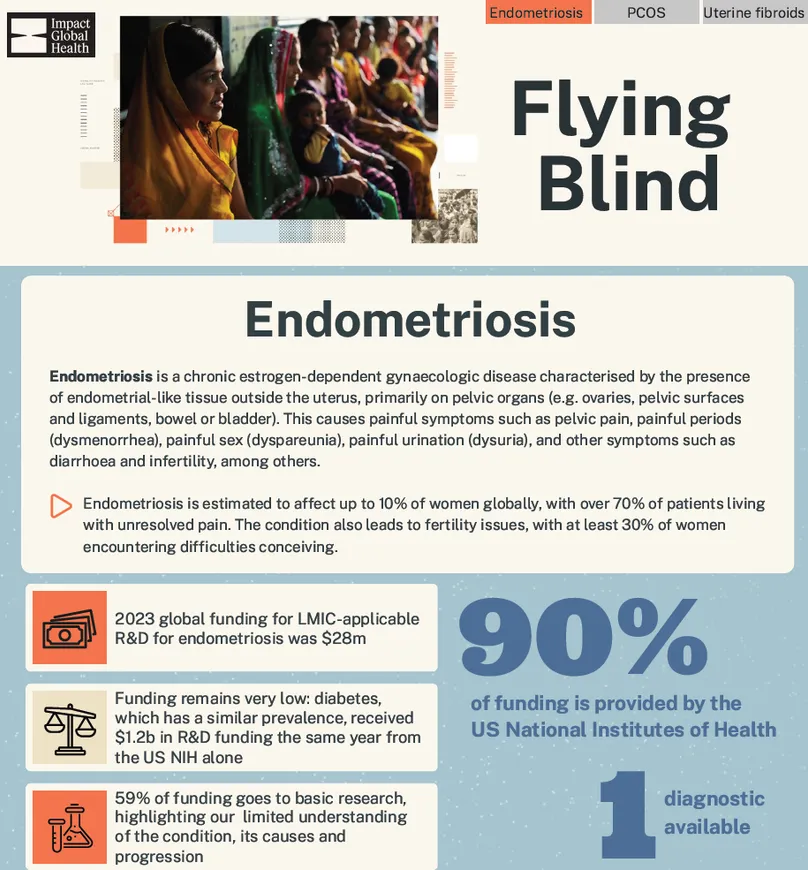
Endometriosis is a chronic estrogen-dependent gynaecologic disease characterised by the presence of endometrial-like tissue outside the uterus, primarily on pelvic organs (e.g. ovaries, pelvic surfaces and ligaments, bowel or bladder). This causes painful symptoms related to the location of this tissue, such as pelvic pain, painful periods (dysmenorrhea), painful sex (dyspareunia), painful urination (dysuria), diarrhoea and infertility, among others. Endometriosis is estimated to affect up to 10% of women, with over 70% of patients living with unresolved pain. The pathology also leads to fertility issues, with at least 30% of patients encountering difficulties conceiving.
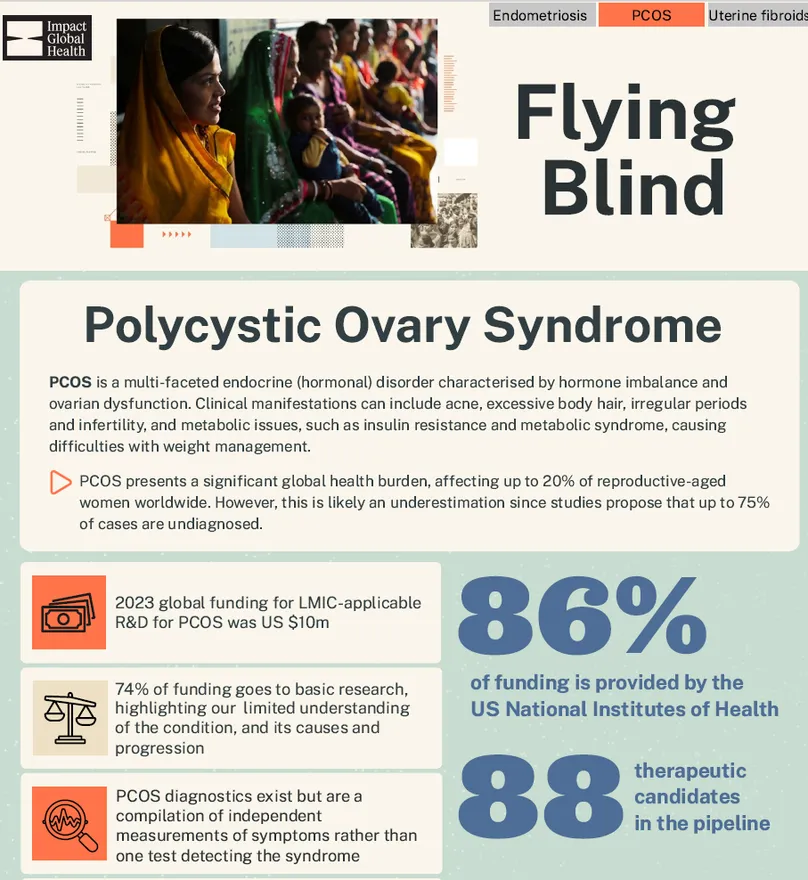
PCOS is a multi-faceted endocrine (hormonal) disorder characterised by hormone imbalance and ovarian dysfunction. Clinical manifestations can include acne, excessive body hair (caused by high androgen – male hormone – levels), irregular periods and infertility (due to infrequent or irregular ovulation), and metabolic issues, such as insulin resistance and metabolic syndrome, causing difficulties with weight management. PCOS presents a significant global health burden, affecting around 20% of reproductive-aged women worldwide. However, this is likely an underestimation since studies propose that a majority of PCOS cases go undiagnosed (68-75%).
Uterine fibroids, also known as leiomyomas or myomas, are the most common benign tumours of the female reproductive system. They affect women differently based on their size, number, and location within the uterus. Pelvic pain, ranging from mild discomfort to severe pain interfering with daily life, is experienced by 20-40% of women, often due to the pressure of the fibroids on surrounding organs or when the fibroid breaks down. About 30% of women with fibroids experience abnormal bleeding, including heavy periods (menorrhagia), prolonged periods, or bleeding between periods (metrorrhagia). Fibroids can also affect fertility, cause obstetric complications, painful urination, pain during sex, abdominal distention, and gastrointestinal symptoms like constipation. Uterine fibroids are estimated to affect 20-70% of women globally, and studies in the US have shown a three-times higher prevalence in Black women, suggesting ethnic disparities in developing the condition.
Impact Global Health curates the gold standard resource in annual global health R&D funding – G-FINDER – alongside up-to-date product pipelines unmatched in their detail and coverage. Leveraging our data, this report provides a critical and timely landscape analysis of global R&D for these serious women’s health conditions in two parts: by evaluating and contextualising current investment, and by reviewing and analysing the pipeline of biomedical products developed and used to diagnose and treat them. By understanding what is being spent and researched, we hope the sector will be better placed to direct energy and resources towards initiatives with greatest promise and impact, accelerating progress in women’s health and wellbeing.
Funding data was collected through our G-FINDER survey. Pipeline data was extracted from diverse sources including pharmaceutical databases, clinical trial registries, scientific literature and funders’ grant databases following our pipeline methodology. We searched these databases for drugs, biologics, devices and diagnostics in active development, i.e. with evidence of clinical activity in the last three years (2021-2024). The resulting datasets comprised 16,915 individual entries (7023 for endometriosis, 3425 for uterine fibroids and 6467 for PCOS), which were scoped manually to identify and cross-check marketed products and candidates in development. Individual profiles containing information about mechanism of actions, developers, clinical development stages and ongoing clinical trials are accessible on our portal.
1 While we acknowledge that gender identity is complex and non-binary, we use the term ‘women’ and ‘female’ to refer to biological characteristics, such as having a uterus and being capable of pregnancy, that are usually correlated with being perceived as a woman. While not all women have these sex characteristics, and not all people with uteruses are women, the overlap between female sexual and reproductive organs and the social category of ‘women’ is meaningful to categorise how issues specific to this biological sex have been approached culturally and institutionally.
Download the PDF of the reportWhat’s being spent: the rigged race of gynaecological R&D
Funding for endometriosis, PCOS and uterine fibroids for 2023 – our most recent data – is limited when compared to other sexual and reproductive health conditions. Combined, these three conditions totalled $50m, which is 17 times less than the rest of LMIC-applicable R&D for SRH conditions. Although sexual and reproductive health is arguably a women-centric health area to begin with, investment in these three conditions that exclusively affect women accounts for only 6% of the total. It also represents only a fraction of what is spent on other global health issues: for instance, R&D funding for these three conditions combined adds up to only 7% of what was spent for malaria in 2023.
Side-by-side, endometriosis fares better than the other two, with twice as much funding ($28m) as uterine fibroids ($12m) and three times as much as PCOS ($10m), reflecting its higher profile and recent interest around the disease. Our recent analysis of the US National Institutes of Health’s (US NIH) funding for endometriosis also highlighted a five-fold growth between 2017 and 2023, from $6m to $29m. Even with this recent increase, levels of funding do not correspond to the burden of the condition: diabetes, for instance, which has a similar prevalence, received $1.2b in R&D funding the same year. A 2022 study demonstrated that if endometriosis were funded at the same level as diabetes in relation to its annual economic burden in the US, its NIH funding would need to increase to $298.8-$455.3m. Either way, funding for endometriosis and uterine fibroids falls below the level required to meet R&D needs for novel non-invasive diagnostic tools and therapeutics. PCOS received even less funding than both endometriosis and uterine fibroids in 2023 and this level of investment is also unlikely to bring much needed biomedical innovations to the one in five women who suffer from the syndrome.
The neglect of women’s health conditions through limited levels of R&D funding is well documented and affects all conditions in our portfolio that affect women exclusively: maternal health conditions (preeclampsia, postpartum haemorrhage, preterm labour, maternal iron-deficiency anaemia) and gynaecological conditions (endometriosis, PCOS, uterine fibroids and menopause) together received less than 20% of the total investment in sexual and reproductive health R&D in 2023.
Figure 1: Funding for SRH conditions in 2023, in USD millions
Beyond size, the maturity of the R&D landscape for these conditions is also reflected in where investment is focused. For all three conditions, most funding is dedicated to basic research, highlighting our persistent, limited basic understanding of their aetiologies and disease progression. From this knowledge void, product development for these conditions has been inevitably slow off the mark. Still, while basic research represents at least three quarters of R&D funding for PCOS and uterine fibroids (74% and 85.5% respectively), the trend is less pronounced for endometriosis, with only 59% of funding dedicated to basic research and over a third dedicated to the development of biomedical products to address the condition. This might reflect increased momentum and slightly higher levels of recent funding, perhaps providing us with more actionable medical knowledge on the condition to spur product development – even without significant breakthrough in our foundational understanding. Alternatively, the growing attention on endometriosis may be fuelling a much needed push for R&D in the face of imperfect understanding to help improve women’s lives. That said, 73% of the funding for endometriosis medicines – and the totality of diagnostics funding – still goes to early-stage research. While this signals dynamism and the first, necessary, steps for product development, the immaturity of the current pipeline stresses probable delays and failures before any new products reach the market.
Figure 2: Proportion of R&D funding by research/product type and by condition, in 2023
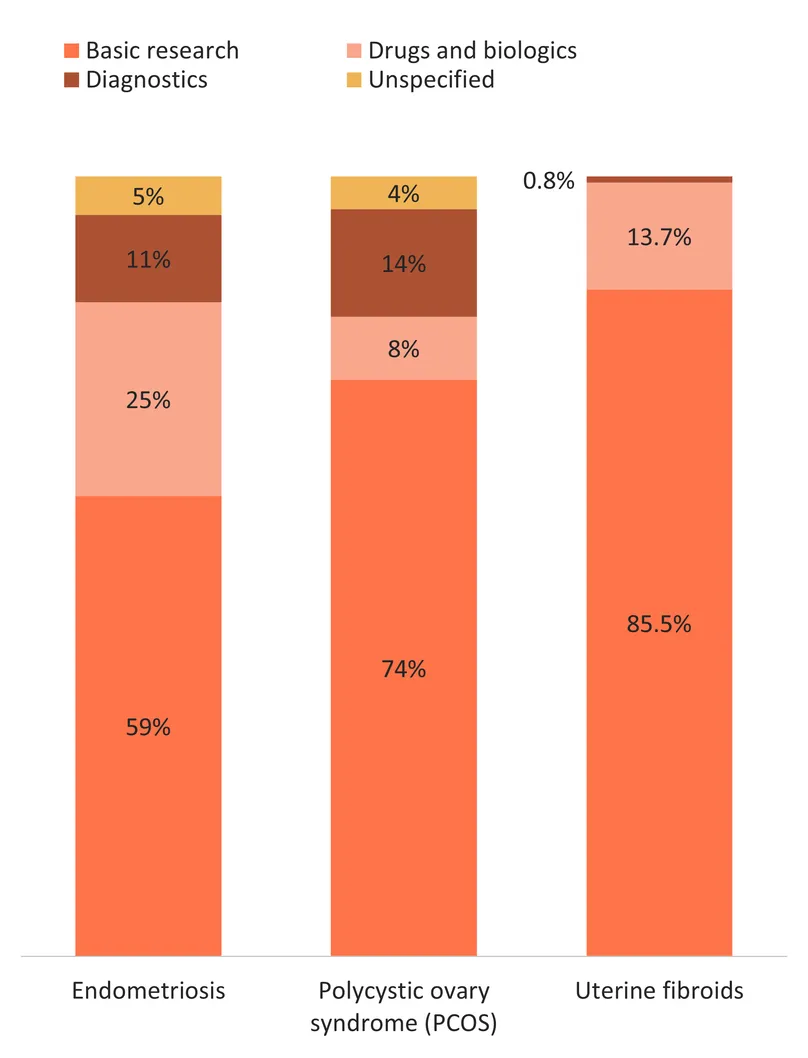
Investment in product development focuses on medicines (drugs and biologics) for both endometriosis and uterine fibroids (25% and 14% of total funding respectively), while diagnostics for PCOS received more funding than drugs (14% vs. 8%). Despite the urgent need for diagnostics for endometriosis, funding for this product category represented only 11% of the total, which is mirrored in a rather limited and nascent pipeline. There was no investment reported for uterine fibroid diagnostics R&D, with a non-existent pipeline for this product category which is concerning as ultrasound isn’t accessible to all.
Nearly all funding for all three conditions came from public sources, with no industry investment reported. This may reflect gaps in our data, but no industry survey participants reported R&D funding for these three conditions. Lack of industry interest in women’s health issues is not new, and has many reasons including an institutional reluctance to conduct clinical research with pregnant or potentially pregnant women, social and medical dismissal of women’s pain, perceived low return on investment and historical undervaluing of women’s health, including major data gaps, that have led to a focus of clinical research and funding on men.
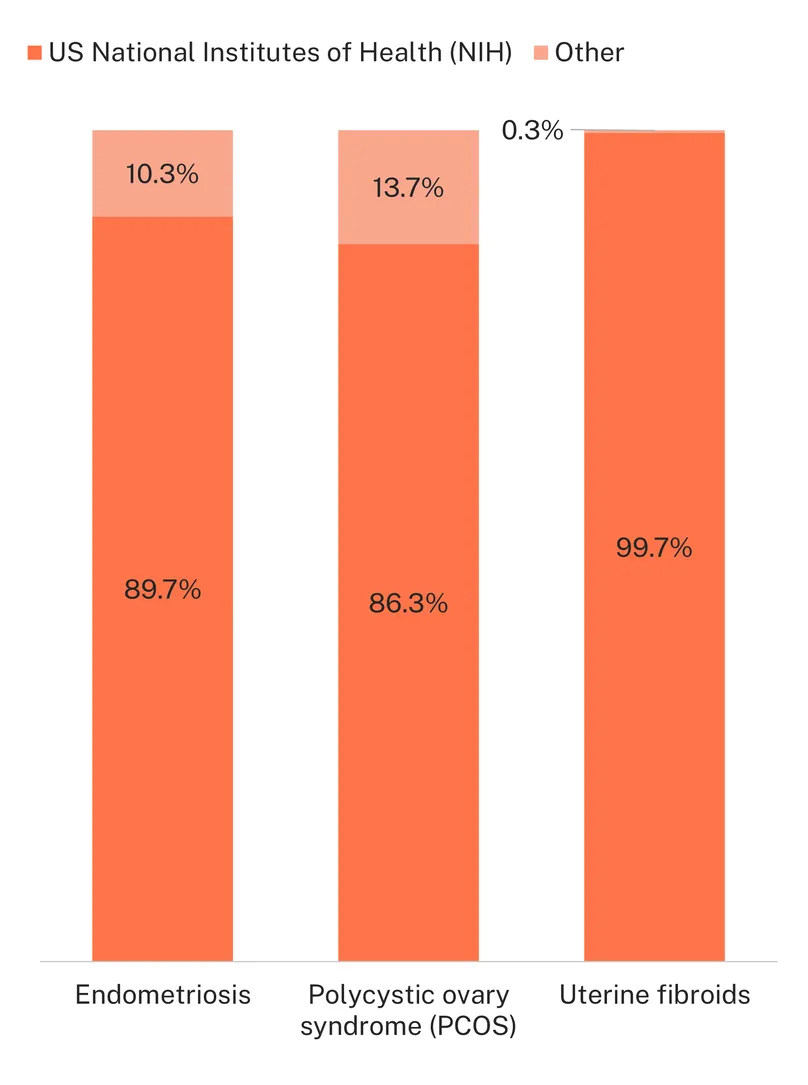
Worryingly, the R&D ecosystem for endometriosis, PCOS and uterine fibroids appears vulnerable, with only 14 funders, 12 of them contributing less than a million dollars. Funding is overwhelmingly dominated by the US NIH, which, with current geopolitical and domestic US policy shifts, signals a concerning dependency that may prove disastrous in the near- and long-term future. Considering current R&D funding levels for these conditions lag far behind other SRH conditions and are unlikely to generate products to meet the needs of women globally, any decrease or cessation in US funding would be catastrophic for the sector if not addressed by other funders.
Figure 3. Proportion of US NIH funding by condition
Unsurprisingly, the underfunding and nascent state of investment in R&D for endometriosis, PCOS and uterine fibroids is reflected in a current lack of innovation in the pipeline, with a predominance of repurposed medicines providing little more than symptomatic management, and limited development of diagnostics.
What’s in the pipeline: band-aid solutions and postponed innovations
Between 2021 and 2024, we identified 387 available, marketed products and active candidates in the pipeline for endometriosis, PCOS and uterine fibroids combined: 106 are existing products, with 281 in the development pipeline. Most products and candidates are medicines, specifically drugs (255, 66%, including 80 marketed products); devices have the fewest (17, 4%, including five marketed products). The smallest pipeline is for uterine fibroids (18 products and 31 candidates in the pipeline) while PCOS has the largest (57 products and 148 candidates).
Product landscapes for endometriosis, PCOS and uterine fibroids
Overall, a quarter of the pipeline consists of marketed products (106, 27%). Most of the these are repurposed (95%), so the number of available marketed products is not a reflection of targeted product development. Among candidates, the proportion of repurposed medicines and devices averages 58% across the different pipelines. Without understanding the cause of these conditions, clinicians and researchers are turning to existing therapeutics for solutions – which is necessary to provide symptomatic relief now – but there are limitations to these treatments, and the pipelines lack true targeted innovation that could offer more to women affected.
Due to the heavy reliance on repurposed drugs, the pipelines superficially appear large. This is further buoyed by the varied and numerous clinical symptoms of the conditions. It does not reflect strong investment or targeted product development. The larger PCOS pipeline points to its higher number of clinical symptoms, including infertility, acne, menstrual cycle irregularities, and metabolic disturbances such as insulin resistance. A variety of repurposed therapeutics can be used to treat these different symptoms, although none have been developed specifically for PCOS, nor do any address the condition’s underlying pathology. For endometriosis, most of the pipeline consists of repurposed medicines and devices providing pain management and halting or slowing the progression of the disease (hormonal medicines), with a limited number developed to address the underlying pathology (i.e. tissue growth outside of the uterus). For uterine fibroids, most therapeutics focus on two symptoms, bleeding management and shrinking fibroid size, which translates to a smaller pipeline.
Figure 4. Medicines for endometriosis, PCOS and uterine fibroids by archetype
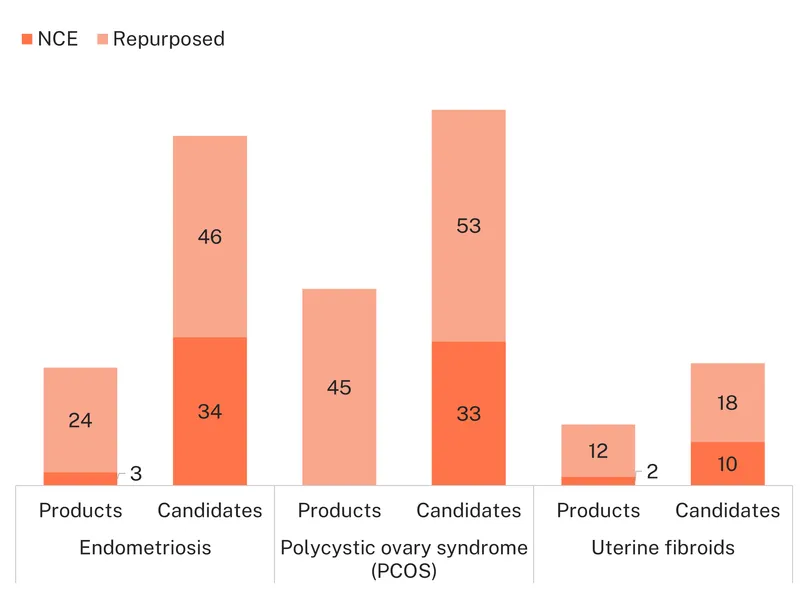
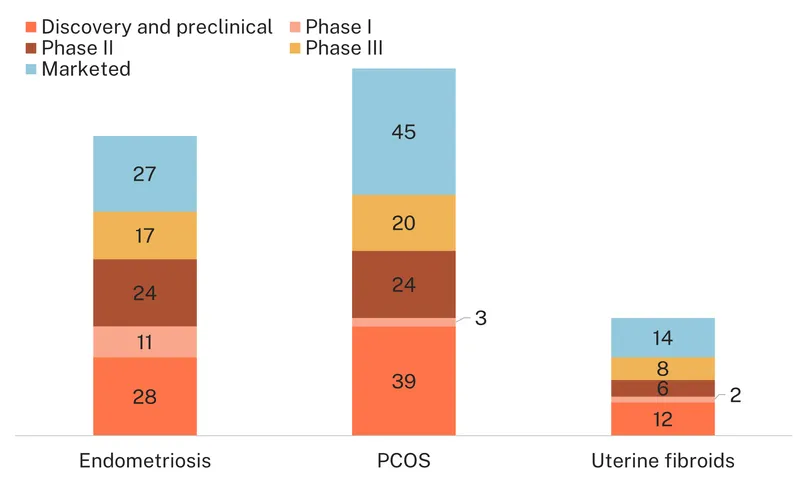
While the focus on repurposed therapeutics is true for all three conditions, the R&D field for endometriosis is a little more innovative, with a higher proportion of new chemical entities or new devices vs. repurposed medicines/devices in the pipeline (47% vs. 38% for PCOS and 40% for uterine fibroids). There is also a higher proportion of candidates in clinical development, as opposed to discovery and preclinical, (65% vs. 55% for PCOS and 57% for uterine fibroids), which signals more progress and is consistent with the higher level of funding captured in the G-FINDER survey.
Figure 5. Medicines for endometriosis, PCOS and uterine fibroids by development stage
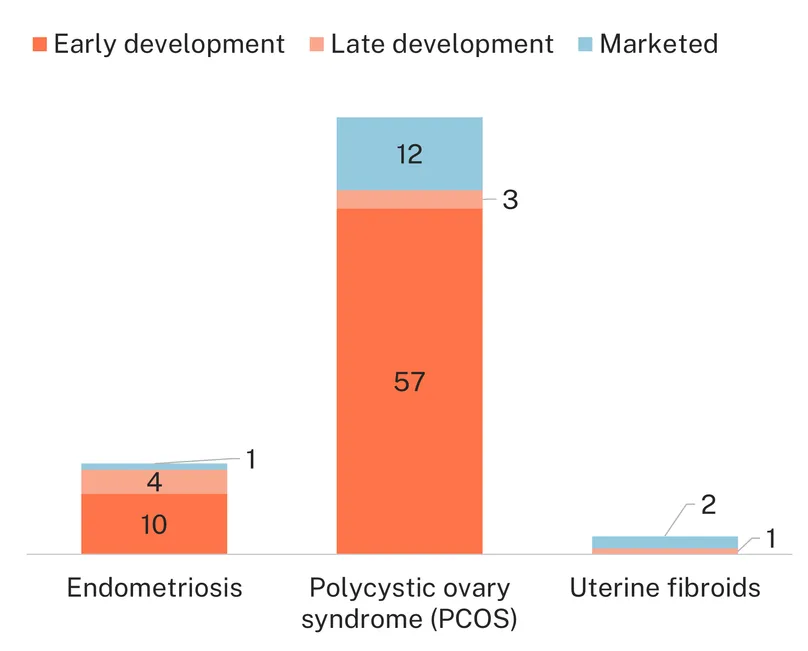
The diagnostic pipeline presents more variety between conditions. Despite the urgent need for less invasive tests, only one marketed test is available for endometriosis, and the pipeline is limited, with only 14 candidates, 10 of which are still in early development. In contrast, the diagnostic landscape for PCOS is more dynamic, with 12 adopted diagnostics, used in different combinations according to the Rotterdam criteria2, and 60 candidates, most of which are based on promising biomarker research. But the pipeline is also immature, with 89% in early development and at risk of attrition. This partially reflects the same phenomenon we see for medicines: given the complexity of PCOS and the diversity of its clinical manifestations, biomarker research aims to explore a larger number of pathways – including metabolic biomarkers – than for the fewer symptomatic manifestations of endometriosis. The diagnostics pipeline for uterine fibroids is non-existent, with no other diagnostic avenues being explored aside from traditional ultrasound and MRI techniques, which are not always accessible in low-resource contexts.
Figure 6. Diagnostics for endometriosis, PCOS and uterine fibroids by development stage
While each pipeline presents singularities in size and structure which are detailed in the annexes of this report, they all share the structural consequences of a lack of research, limited understanding of their pathologies and chronic underfunding. For all, this results in underdeveloped and/or inadequate therapeutic and diagnostic options.
2 Established in 2003 and revised in 2018, the Rotterdam criteria are the standard diagnostic guidelines for PCOS. They define PCOS as the presence of two out of three criteria: hyperandrogenism (elevated levels of male hormones, assessed clinically -presence of acne, hirsutism- or biochemically), ovulatory dysfunction (oligo/anovulation, infrequent or absent ovulation), and/or polycystic ovarian morphology on ultrasound: either the presence of 20 or more follicles on a single ovary or ovarian volume above 10 cm3.
Discussion: fragile gains at risk
The R&D landscape for endometriosis, PCOS and uterine fibroids paints a clear picture of what happens when there is persistent historical neglect of women’s health in medical research. Levels of investment lag embarrassingly behind other global health issues, even within the relatively women-focused field of sexual and reproductive health. Moreover, most of the little funding that is available is not directed to product development yet. Decades of dismissal have left these conditions poorly understood, meaning over two thirds of R&D funding is being spent on basic research. Although this is a necessary first step in biomedical development, it is also a sign that R&D for these conditions operates in a somewhat rigged race, starting off with – and slowed down by – a lack of funding and available medical knowledge from the outset. Many factors contribute to this institutional neglect, including gender bias in medical and clinical research and institutional and societal dismissal of women’s health – and particularly women’s pain – altogether. As a result, both therapeutic and diagnostic development for conditions that affect at least one out of ten women worldwide are still nascent, leaving millions of women globally with few answers and limited medical options.
Like maternal health conditions such as preeclampsia, lack of understanding of the conditions and their multiple clinical symptoms render diagnosis complex. Current diagnostic options are suboptimal, either because of their limited performance, invasiveness or reliance on procedures and technologies that may be inaccessible in low-resource contexts. For both PCOS and endometriosis, biomarker research holds promises, but most diagnostics in development are in very early stages, which means progress will take a while. Even worse, diagnosis of uterine fibroids entirely relies on imaging technologies with limited access in low-resource settings, and the non-existent pipeline shows nothing new is on the horizon.
On the therapeutic front, there is no cure available for any of these conditions. In the face of limited funding and poor disease understanding, researchers have understandably turned to existing medicines, mainly hormonal and pain medications, for potential quick wins. Current medical management for all three conditions is based on attempts to find solutions from approved symptomatic medications, and while it is positive that women can be offered some options to deal with symptoms that severely impact their quality of life, these repurposed drugs and biologics offer only a band-aid solution, with none addressing the conditions’ underlying causes. As a result, relief is limited and sometimes comes with significant side-effects. While the overall number of therapeutics in use and in development looks encouraging at first glance – particularly for PCOS with 45 marketed products and 88 candidates under investigation – it is largely the result of a diversity of symptoms addressed individually with specific repurposed drugs and biologics, such as antibiotics to treat acne or fertility treatments. True innovation in the therapeutics space is lacking, meaning targeted, curative approaches to stop or reverse the conditions are, as yet, nowhere in sight. Even new chemical entities tend to follow the traditional hormonal avenues for symptom relief, with a quarter of all new drugs being hormonal treatments impacting GnRH or progesterone receptors. Their limited innovative potential will likely reduce the prospective of any vast improvements over existing options.
Most of the repurposed drugs and biologics used to treat either of the three conditions have been developed for other gynaecological indications, such as contraception, menopause management and hormone-dependent cancer treatments. Thirty-four are indicated for at least two of the three conditions covered in this report, and seven indicated for all three, which reflects a gynaecological path dependency rather than causal commonalities between the conditions. Diagnostic research, however, appears to be more specific in its approach, as illustrated by an absence of crossover -particularly in biomarker research – between conditions (with the exception of the anogenital distance measure investigated for both PCOS and endometriosis). Bespoke biomarker research could be the key to more innovative approaches and a first step towards developing products to address the underlying causes of the pathologies and provide, if not a cure, at least tangible and long-lasting relief.
Realistically, the underdevelopment of basic research presents a sizable obstacle to developing true innovations. But the case of endometriosis demonstrates that product development can progress even in the absence of complete disease understanding, with promising results. Benefitting from momentum in the past years, the condition received two to three times more funding than uterine fibroids and PCOS in 2023 ($28m vs. 12 and 10 respectively). While limited, its pipeline appears more innovative than for the other conditions, with 47% of medicines being new chemical entities (versus. 38% for PCOS and 40% for uterine fibroids), and more advanced, with a higher proportion of candidates in clinical development. This agility and impact with just modest funding increases and targeted initiatives, shows what can be done even in the face of persistent knowledge gaps. Endometriosis could prove an important test cast, paving the way for other neglected women’s health conditions.
Recent wins in endometriosis show that change is possible for women’s health conditions, with the support of strong advocates. The FemTech sector – especially - offers a powerful example of targeted, organised women-centred advocacy and R&D emerging in response to the institutional medical neglect of women’s health issues, with a promising high impact future. However, there are worryingly few current funders in the gynaecological R&D space, with a heavy reliance on support from the US government. The US NIH alone is responsible for between 86% and 99% of all global funding for the three conditions. Considering recent US policy changes away from global health, women’s health and gender-sensitive research, any funding cuts – sizeable or not – will endanger the small progress made in the field and further postpone much needed biomedical products making it to the market. In the context of geopolitical uncertainty and restricted public research budgets, private capital and the FemTech sector may offer, if motivated, promising avenues of hope. However, the larger women’s health community will also need to remain visible, loud and relevant, if the policies, coordination and funding needed to develop the diagnostic and treatment options women have been forced to live without for too long, are to finally come to fruition.
Detailed insights: landscape for endometriosis
There are many R&D unmet needs for endometriosis: non-invasive point-of-care tests are needed to provide quicker, easier and more accessible diagnosis than surgical exploration. Surgical resection of lesions is a common treatment, but beyond accessibility issues in low-resource settings, it has limited efficacy, and rates of lesion recurrence, repetitive surgical procedures and pain persistence are high. Therapeutics that address the underlying cause to alleviate pain, prevent tissue migration and decrease lesion size or suppress them are needed. But available medicines only offer limited symptom management, and many women live with debilitating pain despite treatment. The current endometriosis pipeline only partially addresses these unmet needs, with most medicines following the symptomatic management path and a current lack of non-invasive diagnostic tests.
The therapeutic landscape for endometriosis consists of 107 medicines, including 94 drugs and 13 biologics. Twenty-seven drugs are marketed products (25% of medicines). Most of them (24/27, 89%) are repurposed, initially developed for either general pain management (e.g. NSAIDs, opioids and pregabalin, an anticonvulsant drug), or as hormonal therapies (24 hormone-dependant medications for cancers and contraception) offering short-term options to reduce pain by halting oestrogen production and endometrium growth. Both focus on symptomatic management. Neither are a cure.
Clinical guidelines currently recommend the use of hormonal drugs developed for contraception as first-line treatment for endometriosis-related pain: 13 of the marketed products fall in this category, though only some have received approval specifically for endometriosis, such as norethindrone and dienogest. A vaginally-administered tablet formulation of micronised progesterone was also developed by Besins Healthcare for the treatment of female infertility, menopausal syndrome, menstrual disorders and endometriosis. While recommended, the use of oral contraceptives for the treatment of endometriosis is mostly off-label and has limited evidence of efficacy, with some studies showing persistence of pain in up to 59% of women. Side-effects such as acne, depression, bloating, irregular, prolonged and breakthrough bleeding, headaches, nausea and weight gain, and an increased risk of venous thrombosis for combined contraceptives can occur. They are not a therapeutic option for women who want to conceive.
Nine approved products are gonadotropin-releasing hormone (GnRH) analogs, antagonists or agonists which were originally developed for the management of hormone-dependant cancers but are now registered and indicated specifically for endometriosis. Three are new chemical entities developed specifically for endometriosis and uterine fibroids: relugolix alone, relugolix combined (with estradiol, and norethindrone acetate), and SHR7280, a GnRH antagonist also investigated for female infertility, heavy menstrual bleeding, and prostate cancer. Danazol, a synthetic steroid with antigonadotropic and anti-estrogenic properties was the first FDA-approved treatment for endometriosis, but its use has been restricted in some regions due to safety concerns around liver toxicity and androgenic and teratogenic side-effects. In general, these treatments also come with side effects ranging in severity, and as such are only recommended if hormonal options have proven ineffective and for a maximum duration of 12 months.
Figure 7. Medicines in use and in development for endometriosis by clinical development stage
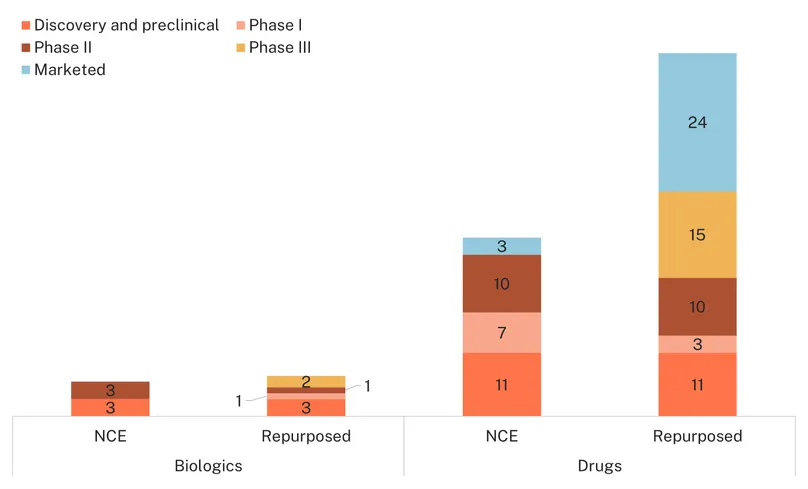
The pipeline for endometriosis includes 80 candidates in development, of which 46 are repurposed medicines (drugs/biologics) and 34 are new chemical entities.
Most drug candidates are in clinical development (45 out of 67, 67%) which is an encouraging sign that investment in basic research has paid off, uncovering therapeutic avenues now being actively pursued. However, the most advanced candidates still tend to be repurposed drugs over novel NCEs: all candidates in Phase III are repurposed and no NCE has progressed past Phase II yet.
Among these dominant repurposed drug candidates, various avenues are being explored. Nine are drugs developed to decrease oestrogen production for hormone-dependent conditions. These include four GnRH agonists/antagonists or analogs, estrogen receptor modulators (raloxifene), and aromatase inhibitors (letrozole). Others are drugs marketed for psychiatric indications, such as ketamine, citalopram, esketamine, N-acetylcysteine, or for neurological conditions like epilepsy, Parkinson’s or neuropathic pain (for example, cannabidiol). Anti-inflammatory drugs, such as prednisolone and quercetin, are also being explored, as well as general class antibiotics. The diversity of repurposed drugs under investigation is positive as different pathways are being explored, and it is a practical step towards meeting women’s needs sooner – though none yet address the underlying causes of the condition.
Among the most advanced NCE drugs (Phase II), several target GnRH (three) or progesterone (one). However, new avenues are also being explored such as FOR-6219, a novel small molecule inhibitor of 17β-Hydroxysteroid Dehydrogenase 1 developed by Organon, which blocks the formation of estradiol, reducing endometriotic lesions and pain without affecting natural hormone or ovarian function. Another example is DLBS1442, a bioactive fraction from Phaleria macrocarpa Boerl fruit, which has demonstrated dose-dependent inhibition of cell migration and antalgic effects.
Thirteen biologics were identified, with an even split between preclinical and clinical research. As with drugs, the most advanced biologics are repurposed: follitropin delta and follitropin alpha are routinely used in fertility treatments, and evaluated for endometriosis patients in Phase III studies, but not beyond this indication. New biological entities are either in discovery and preclinical (three) or in Phase II (three), including HMI-115, a prolactin receptor monoclonal antibody and MT 2990, a monoclonal antibody targeting interleukin-33 to treat pain. While the latter are truly innovative, the accessibility of monoclonal antibodies poses specific challenges in LMICs despite recent progress.
Devices approved for the treatment of endometriosis (three) are repurposed hormonal drug-delivery systems: the contraceptive levonorgestrel IUS and etonogestrel-based implant, and a goserelin (GnRH agonist) implant, first approved for the treatment of hormone-dependent cancers.
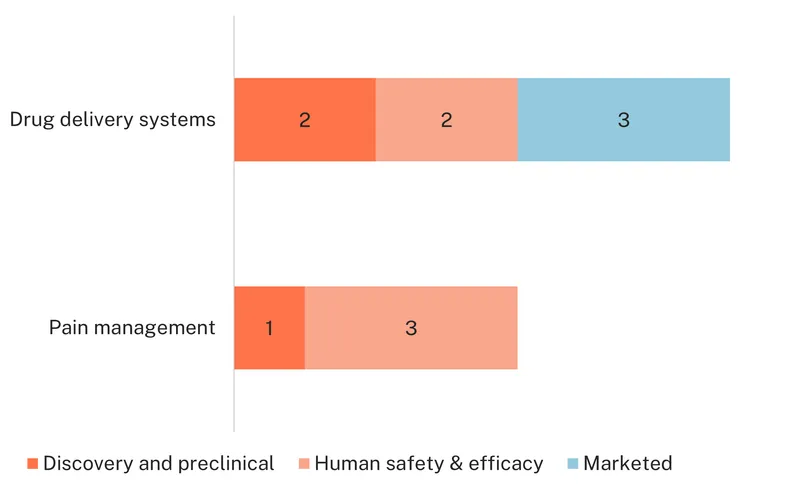
There are eight devices in the pipeline, one of which is a repurposed quinagolide-releasing vaginal ring, marketed for the treatment of pituitary gland tumours, which has showed positive results in reducing endometriosis lesions and improving pain symptoms in the human safety and efficacy phase. The rest are new devices developed specifically for the treatment of endometriosis. Three are drug delivery systems: the progestin vaginal ring (VR 103) in human safety and efficacy trials, and two in early stage – the anastrozole vaginal ring and the Womed Leaf – the latter a polymer intrauterine device designed to prevent adhesions currently developed to add a non-hormonal undisclosed drug component, ReLeaf, to treat endometriosis-related pain.
Four devices are developed for homecare pain management. These include the Angel Touch device, a portable magnetic field irradiation tool; Endocare, a digital therapeutic solution to alleviate chronic pelvic pain through virtual reality technology; and Hyivy Health Floora, a device targeting hypertonic pelvic floor disorders with heat therapy and auto dilation. Another device in preclinical development also uses targeted heat therapy, the RICAT (Radiometry Integrated Contrast Applied Therapy) system developed by H3Pelvic Therapy Systems, which presents as a portable, tampon-sized thermal transfer device with a specially designed probe that combines heatpipe technology with radiometric sensors to deliver and monitor precise cooling and heating cycles to the pelvic region.
Figure 8: Devices for endometriosis by product type and clinical development stage
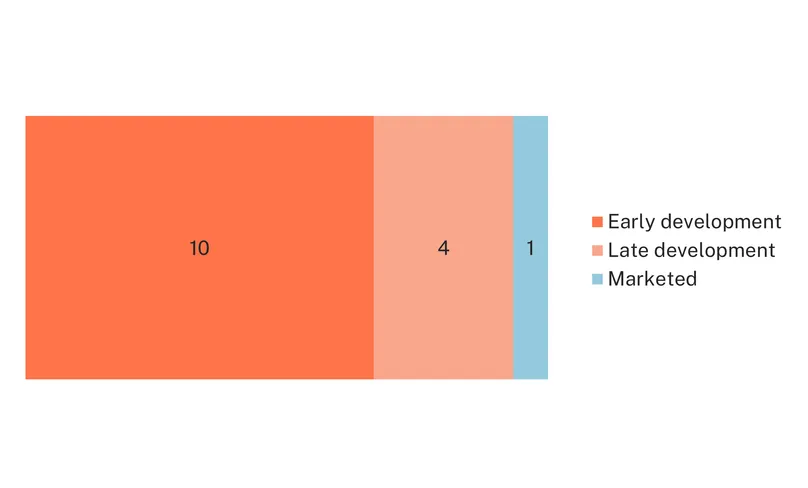
Despite the urgent need for diagnostics beyond laparoscopy, diagnostic research for endometriosis is relatively limited and mostly in early stages. There is only one diagnostic marketed for endometriosis, Ziwig’s Endotest, a saliva-based diagnostic which detects a signature of 109 miRNAs identified through artificial intelligence. The test, which very recently received CE certification and is available in several European and middle eastern countries, is the first non-invasive approved test for endometriosis. The rest of the landscape consists of 14 diagnostic candidates in the pipeline: 10 in early development, and only four in late stage.
The majority of diagnostics are biomarker based, with one individual biomarker test, She Sense, developed by OnaWave Medical, and ten based on multiple biomarker detection. Out of the ten biomarker diagnostic candidates, six are in early development and four in late development, with studies evaluating their diagnostic performance. Biomarker-based tests rely on a variety of samples with the aim of reducing the invasiveness of laparoscopy-based diagnosis: six tests use blood specimen, one uterine tissue collected via pap smear (MetriDx), the two tests developed within the SENSOPAD project (SensoMFgFET and SENSOPAD) use menstrual fluids, and one relies on saliva samples (Endotest).
Only two diagnostic tests in development are based on imaging technologies, both in early development: the MRI contrast agent SpagoPix (SN132D), developed for tumour selective MRI, is currently being tested for the detection of endometriosis lesions, and 3D/4D transperineal ultrasound is being investigated for the detection of deep infiltrating endometriosis via measurements of the pelvic floor muscle.
Two tests are based on visual examination of the anogenital distance (AGD), with several studies finding an association between shorter AGD and endometriosis. This parameter is also used in a multiparametric screening tool developed by the University of Melbourne to rule out endometriosis based on AGD and medical history. Both are in early development.
Figure 9: Diagnostics for endometriosis by technology type
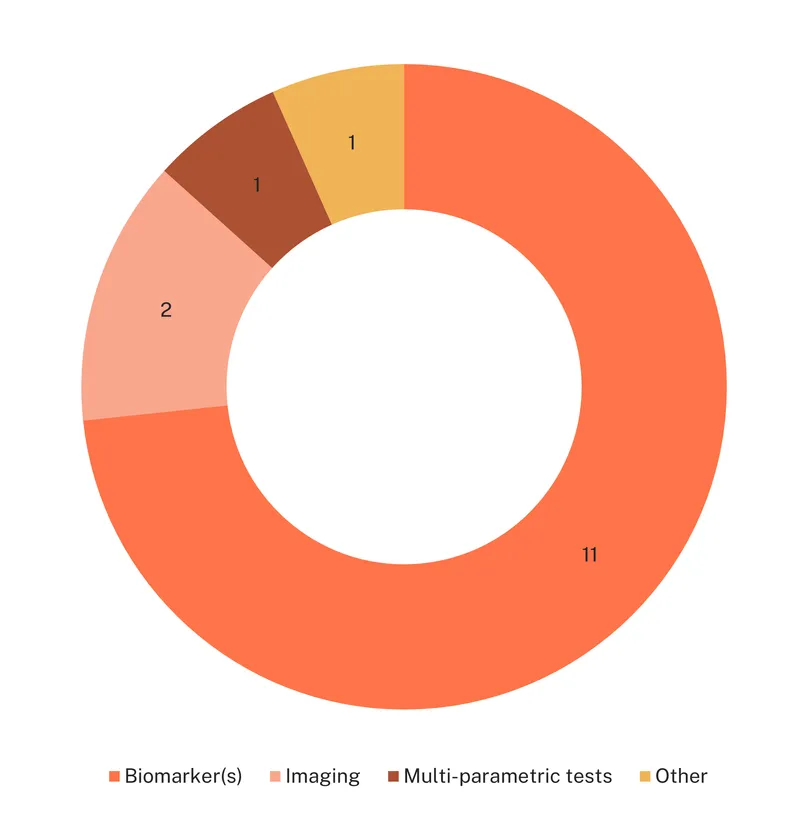
Figure 10: Diagnostics for endometriosis by development stages
Detailed insights: landscape for polycystic ovary syndrome
Further biomedical research is needed for PCOS. Current diagnostics lack consistency and accessibility, and all available treatments are repurposed medicines limited to symptomatic management. The PCOS product landscape is the most extensive of the three conditions, with 131 medicines (including 45 marketed), two devices and 72 diagnostics (including 12 marketed products). However, rather than reflecting sustained R&D efforts for the development of PCOS-specific products, the number of marketed products and candidates in development points towards a fragmented, symptomatic-based approach characterised by a high number of repurposed products, many used off-label, to address this multi-faceted but poorly understood condition. This symptomatic approach is necessary and needed in the absence of a definitive treatment, but both treatment and diagnosis would benefit from an improved understanding of the pathology and its stratification in more precise ways.
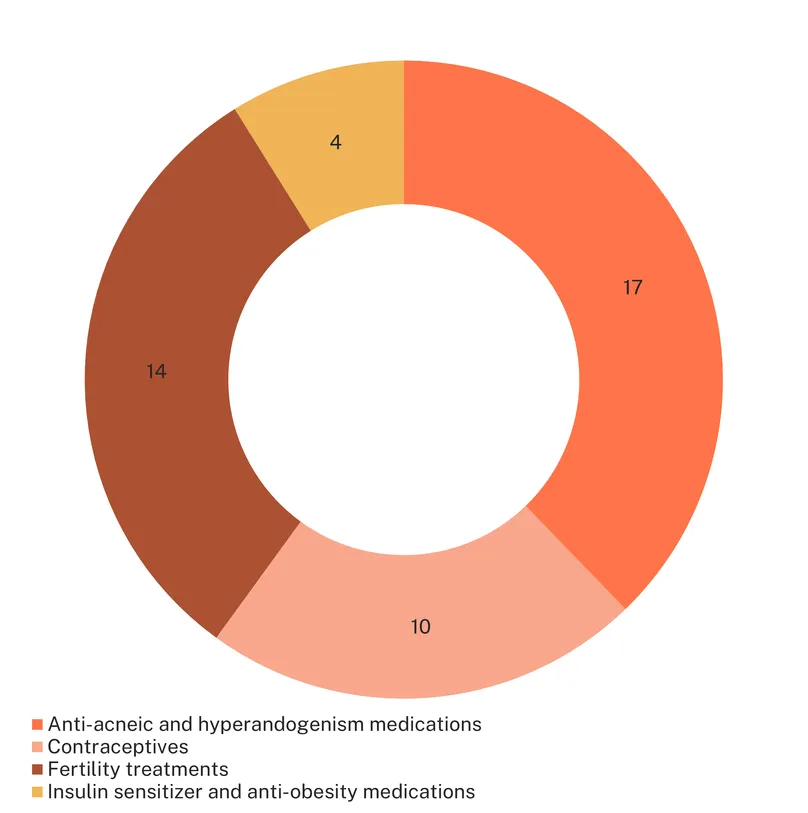
A total of 131 medicines feature in the medicines landscape for PCOS, including 45 marketed products and 86 candidates in development. Marketed medicines for PCOS are mostly drugs (39 drugs versus six biologics), all repurposed. Because of the complexity of the pathology and the diversity of symptoms, these marketed drugs address different aspects of PCOS. While some of these drugs are actually used on-label to treat the symptom they are registered for (e.g., infertility, acne), we consider them here as repurposed since they are not a PCOS treatment and do not address the underlying cause of the condition.
There are 12 drugs approved or adopted for the treatment of PCOS-related acne (due to hyperandrogenism – the over production of male hormones), including eight antibiotics and four retinoids. Hyperandrogenism can also be treated with anti-androgens such as bicalutamide, a non-steroidal androgen receptor inhibitor, and finasteride, marketed for benign prostatic hyperplasia and alopecia. Eflornithine, a medication developed for African trypanosomiasis, is also indicated to treat facial hirsutism.
Hormonal contraceptives are also widely used for the management of hyperandrogenism symptoms and to improve period regularity (which is important to minimise the risk of endometrial cancer). These (10) include progesterone, as well as combined oral contraceptives, notably combining ethinylestradiol with a diversity of progestins (including dienogest, specifically for PCOS-associated hyperandrogenism). However, their use is associated with side-effects, including potentially worsening PCOS-related metabolic disturbances such as insulin resistance. They are also (except progesterone) not a treatment option for women trying to conceive.
Fertility is a prominent indication of drugs and biologics adopted for PCOS treatment. There are 14 drugs and biologics routinely used for fertility treatment that are indicated for PCOS patients trying to conceive. These include biologics such as urofollitropin, follitropin alfa, beta and delta, and human chorionic gonadotropin, amongst others. Additionally, GnRH focused drugs, such as triptorelin and cetrorelix, are recommended to avoid ovarian hyperstimulation syndrome in fertility treatments and improve outcomes.
While research on PCOS has historically focused on sex hormones, metabolic aspects have grown in interest since the 1980s. A few medications have been repurposed to address metabolic disturbances, such as the insulin sensitiser metformin, widely used for the treatment of PCOS since the 1990s. The drug has shown benefits not only on metabolic parameters such as insulin sensitivity, but also for ovulatory function and hormonal regulation, albeit with high variability in efficacy between patients. Anti-obesity drugs, such as liraglutide, orlistat and semaglutide are also used off-label.
Figure 11: Type of repurposed medicines approved or adopted for PCOS
While none of these medicines have been developed for the treatment of PCOS, their combination can be specifically indicated for the condition, not only to simultaneously address a cluster of symptoms, but also for their synergistic impact. For example, the combination of metformin with combined oral contraceptives has shown enhanced benefits on hormonal and metabolic parameters compared to either treatment alone. Adding metformin to hormonal fertility treatments can also improve their efficacy in PCOS populations.
Figure 12: Medicines in use and in development for PCOS by clinical development stage
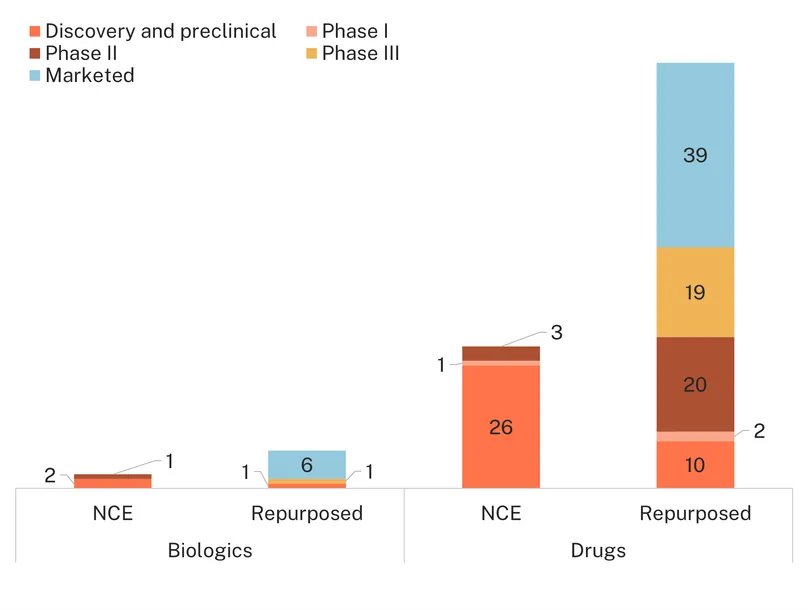
Additionally, there are 86 investigational medicines in the pipeline, including 81 drugs and five biologics. The majority are repurposed (62%, 53/86), following therapeutic avenues already pursued via adopted products (metabolic and hormonal), but there is also a great variety of alternatives, illustrating a ‘stab in the dark’ approach in the face of a poorly understood pathology.
Over a third are investigated for their impact on metabolic function (37%, 32/86), with a quarter of all medicines in development for PCOS antidiabetics (21) often trialled in comparison or combination with metformin. For example, the combination of metformin and liraglutide is being investigated for both metabolic parameters and fertility outcomes in late-stage clinical trials (Phase III), alongside eight other antidiabetics in Phase III. Other repurposed metabolic medicines include phentermine/topiramate (Phase III), the statins atorvastatin and simvastatin and the hyperlipidemia medication, saroglitazar (Phase II). Unsurprisingly, several new chemical entities in development for metabolic diseases (obesity, diabetes, metabolic syndrome) are also being investigated for PCOS, the most advanced being licogliflozin and dichloroacetate, both in Phase II.
Hormone-based drugs are also under investigation, including those routinely used in gynaecology (10), such as GnRH antagonists, like elagolix or ganirelix. Medroxyprogesterone acetate, the selective estrogen receptor modulator tamoxifen, the GnRH agonist tripraline and oxytocin are all of interest for their benefit in fertility treatments (Phase III and II), while the non-hormonal menopausal medication fezolinetant is being tested for its regulating benefits on hyperandrogenism and ovarian function (Phase II). Alternative approaches are also being explored to improve fertility outcomes, such as aspirin (Phase III), the osteoporosis drug alendronate (discovery) or the neurologic medication cabergoline to prevent ovarian hyper syndrome (Phase III).
Recent research has highlighted the association between PCOS and low-grade chronic inflammation, which is reflected in the investigation of eleven drugs for their anti-inflammatory properties. These include the glucocorticoid dexamethasone (Phase III) and the NSAID salsalate (Phase II). The phlebotonic daflon is being tested in Phase II for its impact on inflammation and in animal models for reproductive outcomes and the antioxidant glutathione is tested in rat model in combination with metformin and Diane-35. Additionally, seven NCEs primarily developed for their anti-inflammatory role are in discovery and preclinical for PCOS.
Perhaps illustrating the lack of understanding of the pathology, a number of drugs marketed for a diversity of indications are also being tested for their potential benefits, such as antimalarial drugs, (dihydroartemisinin and hydroxychloroquine), antihypertensives, antibiotics, the anticoagulant enoxaparin (in Phase II), or the opioid antagonist naltrexone (phase III for fertility).
Very few investigational medicines have indications for all the clinical manifestations of PCOS, although melatonin combined with other therapeutics or supplementation (metformin, magnesium) has shown some benefits across the whole spectrum of symptoms. Another promising hypothesis associates PCOS with elevated levels of cortisol: the selective 11β-HSD1 inhibitor BVT.2733 (discovery and preclinical) has shown benefits for obesity, insulin resistance, irregular estrous cycles, reproductive hormone dysfunction, polycystic ovaries, ovulatory dysfunction and subfertility.
Only five biologics are under investigation for the treatment of PCOS. This includes the standard fertility treatment corifollitropin alfa, tested in Phase III, and two monoclonal antibodies in preclinical phases: HAT 002, and the anti-CD19 antibody (aCD19 Ab). Other interesting biologics under investigation include faecal microbiota transplant (tested in animal models), and umbilical cord mesenchymal stem cell (UCD-MSCs) therapy (Phase I/II), with previous studies demonstrating promising results, including a reported case of complete resolution of PCOS in a young woman after a single intravenous injection.
In addition to medicines, there are two devices in development for the treatment of PCOS, both in the human safety and efficacy stage. The contraceptive etonogestrel/ethinylestradiol vaginal ring is investigated for both hormonal regulation and its impact on metabolic parameters to address broader PCOS-related health risks. Continuous positive airway pressure therapy has been investigated for its potential metabolic (insulin resistance) and reproductive benefits in PCOS patients with obstructive sleep apnea (a common comorbidity).
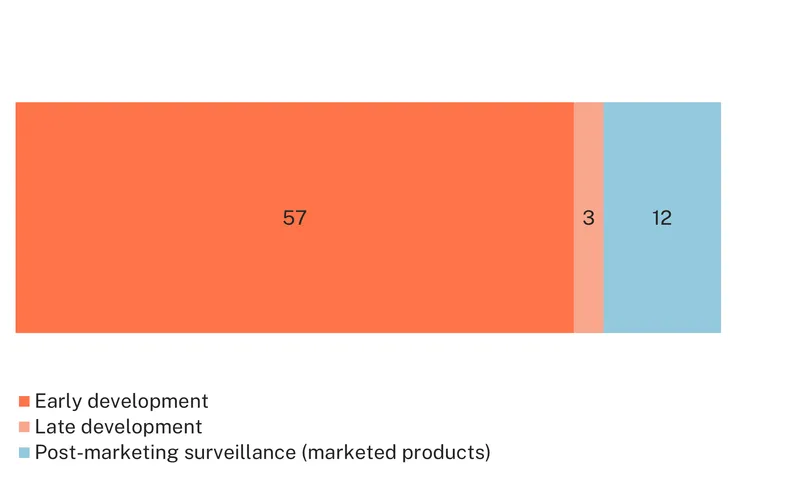
There are 12 marketed diagnostics for PCOS, directly linked to the Rotterdam criteria, which include infrequent or absent ovulation, elevated levels of male sex hormones (hyperandrogenism), and/or ≥20 follicles in a single ovary or ovarian volume >10 cm3. Ovarian volume and follicle count are measured via ultrasound. Hyperandrogenism is typically measured via several biomarker tests (nine), the most commonly used being the free androgen index, which is a ratio of total testosterone to sex hormone-binding globulin (SHBG), with elevated levels associated with PCOS. A clinical assessment of hyperandrogenism can rely on a modified Ferriman-Gallwey score designed to measure hirsutism, where a high score is associated with elevated free testosterone, but this test is limited to one symptom which is not specific to PCOS.
While the Rotterdam criteria are widely used for PCOS diagnosis, their development was based on expert meetings rather than evidence-based treatment guidance, and they do not incorporate clinical manifestations that might be essential to distinguish phenotypes, such as metabolic parameters. There is a need for precision diagnostics that reflect all relevant symptoms, are more effective at predicting prognosis including for fertility and cardiovascular risks, and potentially indicate physiological pathways relevant to the syndrome.
There are currently 60 novel diagnostics in development, mainly additional ultrasound measurements (eight) and biomarker tests (51). Ultrasound-based candidates explore a range of measurements, including ovarian blood flow, uterine artery indices and ovarian stiffness/elasticity and other tissue characteristics.
But it is biomarker testing that constitutes the bulk of new diagnostic research for PCOS, although 96% of them are in early development. Thirty-four individual biomarkers are being investigated for their diagnostic potential for PCOS, including alternative biomarkers to assess hyperandrogenism, such as dihydrotestosterone, and anti-Müllerian hormone (AMH), due to its correlation with ovarian follicle activity. A specific Anti-Müllerian hormone biosensor is in early development by Indian researchers as a potential diagnostic tool for PCOS.
There are also 17 multi-biomarker tests in early development, including three intended for point of care or at home use: a portable aptamer device assessing luteinizing hormone, an at-home urinary androgen index, and the Woost menstrual blood at-home test kit.
The variety of biomarkers investigated (hormones, proteins, amino acids, lipid profile, immune-related biomarkers, and even fructose, mannose and vitamin D) highlights both the complexity and limited understanding of the condition. Biomarker research, while mostly still at the early stage, has the dual potential to uncover specific pathological pathways and improve the overall understanding of the condition.
The only diagnostic in development that does not rely on ultrasound or biomarkers is the measurement of anogenital distance, a marker of in-utero endocrine exposure also investigated for endometriosis diagnosis (early development).
Figure 13: Diagnostics for PCOS by technology type
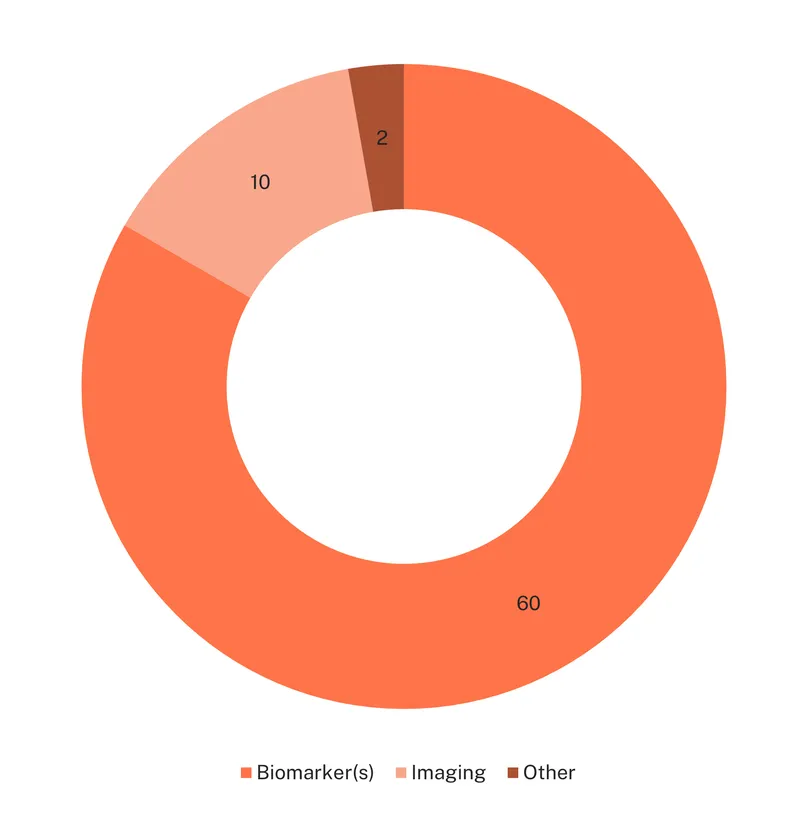
Figure 14: Diagnostics for PCOS by development stages
Detailed insights: landscape for uterine fibroids
While the majority of uterine fibroids remain asymptomatic, cases that are symptomatic can require hospitalization and surgical interventions, such as uterine artery embolisation, or hysterectomy, which has traditionally been the primary approach for the management of uterine fibroids and remains the only definitive treatment available. Diagnosis is based on physical examination, imaging or endoscopy and the lack of understanding of the cause of fibroids seems to have prevented research into biomarkers or other diagnostic tests with more accuracy. There is also a need for medicines preventing the formation and growth of fibroids, reducing their size or supressing them, but current options beyond surgery do not provide definitive treatment. The landscape for uterine fibroids appears very limited, with only 42 medicines (including 14 marketed), four devices (two marketed) and three diagnostics (two marketed), highlighting a lack of investment and research also reflected by the limited amount of funding captured in the G-FINDER survey.
Despite there being 14 marketed medicines available for uterine fibroids, there are none that treat the underlying cause – or offer a cure. Therapeutics available provide temporary symptomatic relief by reducing or suppressing blood loss and reducing fibroid size to reduce pain.
Twelve of these 14 marketed medicines are repurposed drugs. Most of them are contraceptives (such as combined and progesterone-only contraceptive pills, ulipristal acetate and mifepristone), or GnRH antagonists (seven) reducing fibroid-related bleeding and anaemia, uterine volume and fibroid size. The latter includes five repurposed drugs (relugolix, goserelin, elagolix, triptorelin, leuprorelin depot) and two new chemical entities or combinations that have been developed specifically for uterine fibroids: relugolix combined tablet and linzagolix. With the exception of relugolix combined tablet, their use presents the risks of side-effects associated with GnRH agonists/antagonists (hot flashes, sleep disturbances, vaginal dryness, depression and loss of bone mass) and clinical recommendations encourage the use of add-back therapies to mitigate them, such as tibolone. Because of this, they are mostly recommended as a temporary preoperative treatment. All seven GnRH antagonists are officially registered for the treatment of uterine fibroids, while tibolone is used off-label as an add-back therapy. Both GnRH and hormonal contraceptive therapy cannot be used by women trying to conceive.
Non-hormonal treatments include NSAIDs for the management of fibroid related pain and with a potential beneficial impact on blood loss. The antifibrinolytic agent tranexamic acid is a first-line non-hormonal therapy for heavy menstrual bleeding, but not without some risks, notably venous thromboembolism.
The efficacy of these treatments is limited, with inconsistent impact on fibroid size and bleeding, and their use is often limited in time due to their safety profile. New therapeutics are needed to offer women alternatives to surgical resection and improved bleeding and pain symptoms.
Figure 15. Medicines in use and in development for uterine fibroids by clinical development stage
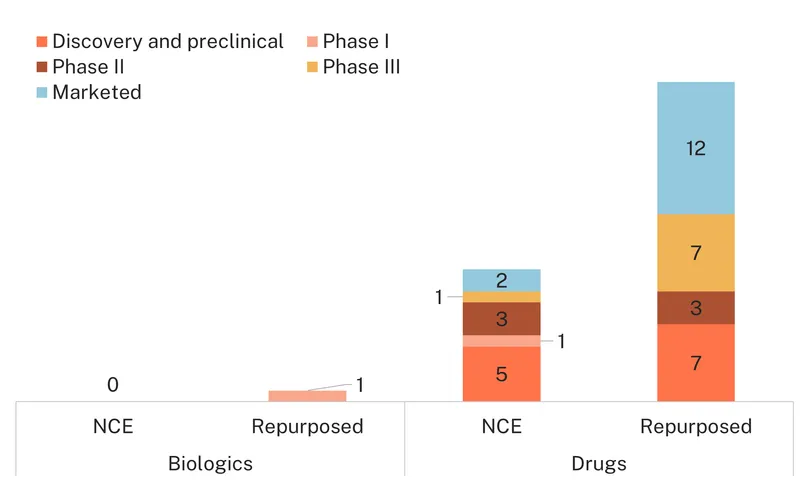
Current candidates in development include one biologic, the repurposed injectable collagenase clostridium histolyticum developed to treat collagen-related conditions and currently in Phase I studies for its effects on fibroids’ stiffness, and 27 drugs, with ten new chemical entities.
Because fibroids are benign tumours, several new chemical entities initially investigated for cancer treatment, such as 2-Methoxyestradiol, are in discovery and preclinical for their potential impact on fibroids. Most other NCEs under investigation follow traditional therapeutic avenues explored for uterine fibroids, which signals limited innovation: there are three GnRH antagonists, including merigolix (Phase II), and a selective progesterone receptor antagonist, telapristone, currently investigated in a Phase II study as a vaginal insert (both also investigated for endometriosis-related pain). SB-UF, a combination of pregnenolone, pyridoxal phosphate and dydrogesterone has also been investigated specifically for the treatment of uterine fibroids during pregnancy (Phase II).
Traditional hormonal avenues of treatment are also explored with repurposed medicines, like progestogens (two), GnRH antagonists (one) and other gynaecological medications such as fezolinetant, marketed for treatment of menopause related vasomotor symptoms (in Phase II), and three drugs initially developed for treating breast cancer (tamoxifen, raloxifene, letrozole).
Two prolactin-lowering agents marketed for hyperprolactinemia and neuropathic conditions, cabergoline and bromocriptine, are investigated off-label for both uterine fibroids and endometriosis, for their potential impact on fibroid and lesion growth and size (Phase III).
Other avenues explored to reduce fibroid size include anti-inflammatory drugs, such as N-acetylcysteine (NAC) (Phase III) and anti-cholesterol drugs like simvastatin (Phase II). Various antihypertensive drugs, such as ramipril and quinapril, and the beta-blocker propranolol, have been associated with reduced uterine leiomyoma incidence in retrospective nested case-control studies, suggesting a protective role and potential therapeutic applications.
There are also four devices in the uterine fibroid treatment landscape. Two repurposed products are approved for their treatment: the levonorgestrel IUS to reduce heavy menstrual bleeding and improve pain symptoms, and the goserelin (GnRH agonist) implant as pretreatment for women undergoing surgical fibroid removal. Just two devices are in discovery and preclinical. One is a raloxifene-loaded drug-eluting insert for intrauterine administration has been tested in animal models. The other is the Womed Leaf intrauterine device, designed to prevent adhesions, which is being developed conjointly for endometriosis and uterine fibroids. For the latter, it is being investigated alongside two specific drugs for uterine fibroids: Fibroid for preoperative fibroid treatment and ReLeaf for alleviating uterine fibroids pain, abnormal bleeding and improving fertility.
There are only two diagnostics currently available for uterine fibroids, both based on imaging techniques: ultrasound (the gold standard), and precision MRI. Additionally, an AI-supported diagnostic tool is being developed by researchers from Huazhong University of Science and Technology to support ultrasound interpretation and uterine fibroid diagnosis. No alternative technical approaches seem to be explored for diagnosing uterine fibroids, including biomarkers, leaving a noticeable gap in the point-of-care diagnostics landscape for this condition. The accessibility of imaging techniques is dependent on resources, which means millions of women might experience symptoms without being able to access diagnosis.
Additional assets
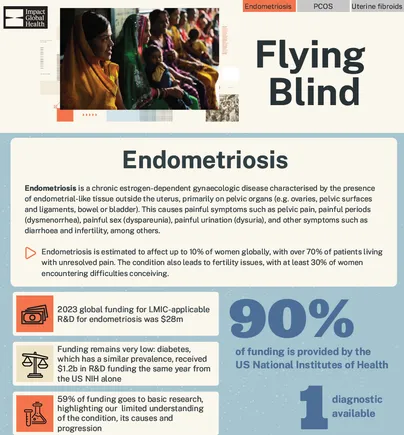
Infographic: endometriosis R&D
This infographic highlights key messages from Flying Blind, focusing on research and development in endometriosis.
Key insights on endometriosis R&D
Watch our short video for an overview of our findings
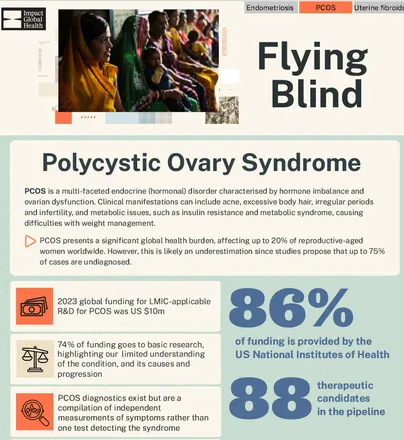
Infographic: PCOS R&D
This infographic highlights key messages from Flying Blind, focusing on research and development in PCOS.
Key insights on PCOS R&D
Watch our short video for an overview of our findings

Infographic: uterine fibroids R&D
This infographic highlights key messages from Flying Blind, focusing on research and development in uterine fibroids.
Key insights on uterine fibroid R&D
Watch our short video for an overview of our findings
Table of contents
- Common yet ignored: the neglect of gynaecological conditions in medical research
- What’s being spent: the rigged race of gynaecological R&D
- What’s in the pipeline: band-aid solutions and postponed innovations
- Discussion: fragile gains at risk
- Detailed insights: landscape for endometriosis
- Detailed insights: landscape for polycystic ovary syndrome
- Detailed insights: landscape for uterine fibroids
- Additional assets