From malaria research to protecting aging populations: AS01 Adjuvant in Shingrix
By Impact Global Health 20 January 2026
Context
Every breakthrough in global health sends ripples that reach far beyond borders. In an era of fiscal tightening and inward-facing policy priorities, investments in global health R&D are under increasing pressure. Yet these investments are among the most powerful drivers of innovation, economic growth, and resilience – not just for low- and middle-income countries (LMICs), but for high-income countries (HICs) as well. Impact Global Health demonstrated that $71 billion in global health R&D funding from 2007– 2023 catalysed $511 billion in GDP growth, 643 000 jobs, and 20 000 patents, a multiplier effect proving that global health investment drives domestic prosperity. The Ripple Effect 2.0 project further examines this dynamic through three case studies of innovations originally developed for LMIC needs that later delivered measurable health and economic benefits in HICs.
This case study focuses on the benefits of AS01 adjuvant, initially advanced through malaria research, before being adopted to enhance the effectiveness of GSK’s ‘Shingrix’ shingles vaccine.
Innovation pathway
The AS01 adjuvant was created as part of global health research aimed at developing vaccines for malaria and was ultimately included in the first approved malaria vaccine, Mosquirix (RTS,S/AS01). Designed to enhance both humoral and cellular immune responses across age groups, AS01 was recognised as an adaptable platform with potential applications beyond malaria. Its performance with other vaccine candidates demonstrated its ability to strengthen immune responses to a range of different pathogens.
Building on this foundation, GSK incorporated AS01 into its recombinant glycoprotein-E shingles vaccine, Shingrix. By pairing AS01 with a non-live antigen, Shingrix was able to achieve a higher and more durable level of protection against herpes zoster (HZ) than earlier shingles vaccines. Approved in 2017, it is currently recommended for adults over 50 and for immunocompromised adults over 18 and is administered as a two-dose intramuscular series. Since its introduction, Shingrix has been adopted in over 50 countries and has contributed to notable reductions in severe shingles and related complications.
The trajectory of AS01, from global health research to a platform now used in vaccines worldwide, demonstrates the potential multi-directional value of investments in global health R&D. Its role in Shingrix, the focus of this case study, and in other products such as Arexvy for respiratory syncytial virus (RSV) and the advancing M72 tuberculosis vaccine candidate, highlight how a single innovation can ultimately help address multiple public health priorities.
- A malaria-driven innovation is now protecting aging populations in high-income countries. AS01, an adjuvant originally developed for malaria vaccines, is now powering Shingrix, the shingles vaccine, now delivering major health gains across the EU, UK, US, and Japan
- By 2050, Shingrix is projected to prevent 32 million cases, save 115,000 lives, and avert 1.2 million DALYs in HICs.
- The societal and economic returns are significant with nearly USD 400 billion in value from healthy years gained and USD 72 billion in health-system cost savings across all four markets.
Health impact in the USA, EU, UK and Japan
We estimate that, by 2050, Shingrix will prevent 32.3 million shingles cases, save about 115 thousand lives, and avert 1.2 million (discounted) DALYs across the EU, UK, the US and Japan. The health impact is largely driven by population size and age structure – the larger and older the population, the more harm done by shingles and the larger the health benefits of an improved vaccine like Shingrix.
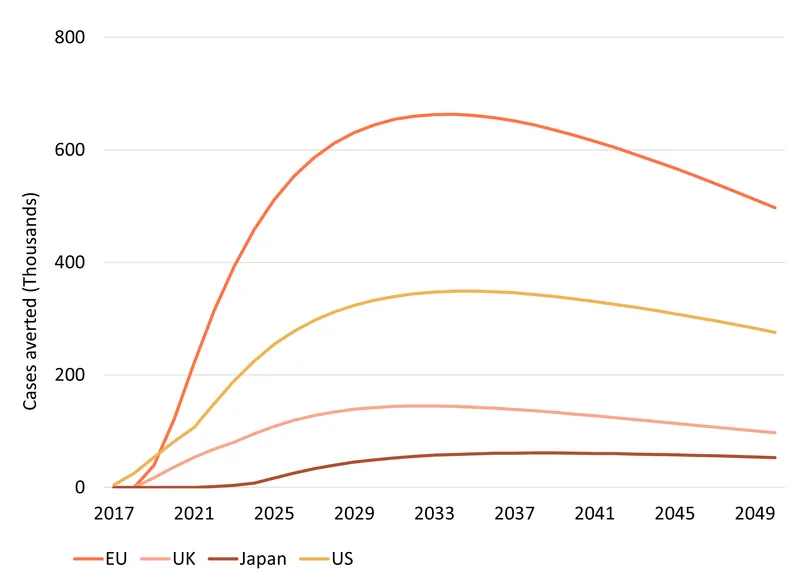
The RTS,S vaccine is estimated to avert 194 million DALYs by 2040 and save 2.2 million lives. While the strength of the comparison is not numeric, the narrative contrast underscores the complementary value: RTS,S reshapes global survival trajectories for children in malaria-endemic regions, while Shingrix reduces one of the most common and costly vaccine-preventable diseases in older adults in ageing, high-income populations.
Economic impact
Being alive and in good health has a value to the individual and to society. Using the Value of a Statistical Life (VSL) approach, which approximates that value mostly based on how people value risky jobs, we estimated the societal gains from 1.2 million DALYs averted to be worth close to US $400 billion1 across our four markets.
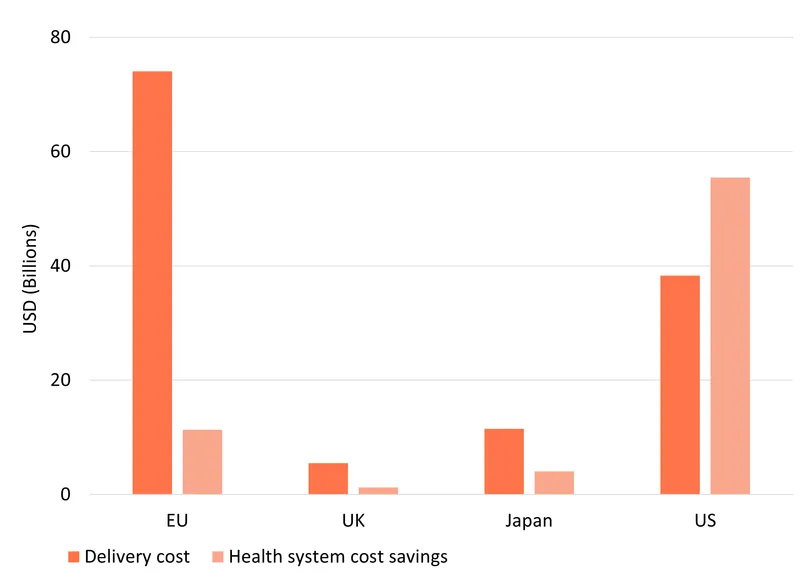
Rolling out Shingrix also generates substantial cost savings for health systems, mainly due to fewer hospitalisations and GP visits. The 32.1 million shingles cases averted, some of which would have developed into complications such as post-herpetic neuralgia, are estimated to result in $72 billion averted health system costs .The largest savings are in the US ($55 billion) because health services and treatments cost more there than in other countries.
Delivering the new vaccine also comes at a cost. We estimate these costs of delivery at $129 billion across our four markets, based on projected vaccine prices and administration costs. In the US, health-system savings outweigh vaccination delivery costs ($55 billion saved from a $38 billion outlay), resulting in a negative incremental cost-effectiveness ratio (ICER) of around $45,000. In practical terms, this means that, each DALY averted in the US through vaccination with Shingrix saves about $44,704 in net health care costs in addition to its major health benefits.
In other markets, ICERs are positive, reflecting a net cost for delivering improved health outcomes. This trade off is particularly common for vaccines, since they typically involve upfront costs to protect health in the long term including, in this case, cases prevented by pre-2050 vaccinations not captured by our 2050 time horizon. We estimate ICERs at $101,110 in Europe, $49,114 thousand in Japan, and $94,637 in the UK. Japan has a lower ICER partly because it has a large and ageing population, who are the most at risk of contracting herpes zoster and developing complications. This implies that averting 1 DALY via Shingrix will generally cost society around $101,110 in Europe, $49,114 in Japan, and $94,637 in the UK, though the true figures, accounting for post-2050 health gains, are likely to be considerably lower
Shingrix is cost-saving in the US and likely cost-effective in Japan as it sees disproportionate benefits due to its large and rapidly ageing population, the group at highest risk contracting herpes zoster and developing complications such as post-herpetic neuralgia (PHN). However, the cost of providing Shingrix, and therefore the ICERs, are very sensitive to assumptions around the spread of coverage and change in price per dose over time, meaning that sharper post-patent price drops than those we have incorporated would further improve the ICERs.
1 For ease of comparison, all monetary values are reported in inflation-adjusted 2025 US dollars
Conclusions
The story of AS01 demonstrates that global health R&D is a cycle of shared innovation and shared returns, not a one-way act of charity. Born from malaria vaccine research and now powering the shingles vaccine, it is projected to prevent millions of cases, save thousands of lives, and avert a great deal of suffering, all while easing pressure on health systems.
AS01 exemplifies the transformative power of global health R&D: a single innovation can save lives, strengthen health systems, and create economic value far beyond its original purpose.
Key assumptions
The projected health impact of shingles vaccination follows clear demographic and epidemiological logic. Vaccine benefits scale primarily with population size and age, meaning that countries with large and/or ageing populations stand to gain the most from vaccination, as the absolute number of individuals at risk of developing HZ and its complications, such as PHN, is substantially higher. The model applies a dynamic cohort approach, where, each year, new individuals age into the eligible populations (either immunocompetent adults 50+ or immunocompromised adults 18+), allowing risk and vaccine-derived health benefits to accumulate across time. In line with the literature, baseline HZ incidence is modelled as increasing progressively with age, and is highest in immunocompromised groups, particularly among organ and stem cell transplant patients. Because shingles can occur more than once, a recurrence rate of 12 cases per 1,000 person-years is applied consistently across both 50+ immunocompetent and 18+ immunocompromised populations. Mortality risk from HZ is assumed to remain very low until around age 80, where case fatality rates rise sharply; A conservative case fatality rate (CFR) of 0.008 is applied uniformly to all immunocompromised patients – the other key group, in addition to the elderly, at high risk of complications from shingles infection. Finally, all future DALYs – including counterfactual future years lived after averted fatalities – are discounted at 2% annually, reflecting standard practice in long-term vaccine cost-effectiveness analyses used by national HTA bodies.
Based on pre-Shingrix clinical practice, the counterfactual alternatives to Shingrix – had it not been developed – included in our model are Zostavax in the EU, US and UK, and an alternative vaccine developed by Biken for Japan. In the no-Shingrix counterfactual we use for comparison, we assume these alternative products proceed as if Shingrix never entered the market. We have assumed 15% coverage for the alternative products – roughly in line with pre-Shingrix trends – and 50% coverage for Shingrix, with a steep rise in the first years after introduction reflecting its much greater efficacy. We model an expanding annual cohort, where, each year, new individuals age into our analysis and are either vaccinated or not based on overall coverage probability. Vaccine durability is modelled through annual waning/decay rates: Shingrix protection against HZ declines only gradually – much more slowly than Zostavax which has been shown to fall rapidly over time. Protection from post-herpetic neuralgia from both vaccines also wanes, but Shingrix again wanes more slowly, reflecting stronger long-term clinical performance.
To calculate the health system cost savings, we have gathered data for each country/region on the healthcare costs of treating HZ and PHN cases. For the EU, we use data from Germany and weighted them to ensure they are representative of the EU average. The cost of rolling out the vaccine is derived from publicly available information, with an assumed 15% decrease in the year following expiry of patent protection, followed by a gradual and smaller annual decline until reaching a floor price set at 55% of its current (market specific) cost. We model both the cost of vaccine rollout and the health benefits delivered through to 2050. However, rollout costs incurred in the final years before our model cutoff will produce almost all their health benefits beyond 2050, meaning that the model includes all costs, but not the long tail of downstream benefits – since patients are typically vaccinated in their early 60s but are not at serious risk of major complications until their 80s. To partly address this time-horizon bias, we have run a sensitivity analysis removing the final 5 years of vaccination costs. As expected, this reduces rollout costs and lowers ICERs: $99,000 in the EU, $48,000 in Japan, $90,000 in the UK, and − $46,000 in the US.
Download a PDF of the case study