The ripple effect: how global health R&D delivers for everyone
By Impact Global Health 22 September 2025
Key messages
1. Investing in global health R&D delivers extraordinary economic returns while advancing health equity
Investing in global health R&D generates remarkable multiplier effects: $71 billion in high-income country (HIC) government funding from 2007-2023 has catalysed $511 billion in GDP growth, created 643,000 jobs, and sparked 20,000 patents. Whilst 90% of this funding has been concentrated in HIC institutions, the investments have delivered life-saving innovations that work across borders. Yet in the current geopolitical climate, traditional funders face pressure to reduce these investments. Scaling back now would not only jeopardise progress in saving lives globally, but it would also forfeit one of the most efficient drivers of domestic innovation and economic growth.
2. Innovations cross borders - with dual benefits for LMICs and HICs
We’ve identified at least 22 health innovations originally developed for use in low- and middle-income countries (LMICs) that have delivered unexpected benefits to HICs. These include vaccines, diagnostics, delivery platforms, and treatments. The AS01 adjuvant developed by GSK, for example, was initially created for the RTS,S malaria vaccine, but has proved pivotal in the development of the Shingles and RSV vaccines, which are now widely administered in HICs. AS01 is also currently being investigated for use in new tuberculosis vaccines, including M72, a potential game-changer in the fight against antimicrobial resistance.
3. Global health R&D is a pillar of national security and builds pandemic response capacity
COVID-19 validated decades of investment in global health research. Platforms developed for malaria (ChAdOx1), TB (GeneXpert), and the rVSV-based Ebola vaccine became essential pandemic tools and helped pioneer a faster approval process. Building distributed research and manufacturing capacity isn't simply about equity - it's about creating resilience for when, not if, the next pandemic emerges from regions with limited surveillance. The speed of our response to the next pandemic will depend on what we invest today. Protecting lives tomorrow means sustaining discovery now.
Interactive map of investment and benefits per HIC
How do HICs benefit from investing in global health R&D?
Global health R&D[1] delivers extraordinary returns for everyone. It saves lives, strengthens economies, and accelerates scientific progress in ways that benefit people in both low- and middle-income countries (LMICs) and high-income countries (HICs). This report builds on a growing body of evidence, including our May 2024 findings showing that every $1 invested in neglected disease R&D generates $405 in societal return.
Between 2000 and 2040, biomedical innovations targeting neglected diseases are projected to save more than 40 million lives and avert 2.83 billion DALYs, generating $49.7 trillion in net societal benefits. Consistent with where the burden of neglected disease falls most heavily, the majority of this impact is concentrated in sub-Saharan Africa and South and Southeast Asia. However, these investments also generate significant health and economic returns in HICs.
Global health innovation is a shared engine of resilience, prosperity, and progress.
In an era of increasing fiscal pressure, where rising defence spending is putting pressure on official development assistance (ODA) budgets and public funding for medical research, it is essential to understand the full value of global health R&D to both LMICs and HICs. Investments by HICs in R&D for neglected and emerging diseases and women’s health are often guided by a sense of global solidarity and shared responsibility. But, beyond these motivations, they are also smart, strategic choices – supporting high-quality job creation, fuelling GDP growth, and strengthening public health systems everywhere. These investments stimulate innovation ecosystems, sustain biomedical industries, and build preparedness platforms that benefit populations globally.
At Impact Global Health, we have consistently demonstrated the life-saving value of biomedical innovation for LMICs. With this report, we broaden our lens to show how smart, sustained investments in global health R&D create returns that are truly global – delivering health and economic progress for HICs. We examine how much government funding from HICs has gone to global health R&D, how much of it stays within HICs, and the macroeconomic and scientific gains it leads to. We then explore the link between R&D and real-world impact, spotlighting case studies that show how innovations developed for LMICs have gone on to help HIC populations, proving that smart, sustained investment pays dividends globally and locally.
[1] In this report ‘global health R&D’ means funding for biopharmaceutical research and development targeting neglected and emerging infectious diseases and women’s health. See our survey scope and methodology for specific details of the diseases, products and conditions included.
Understanding HIC investment in global health R&D
How much have HICs invested in global health R&D?
Data from our annual G-FINDER survey, shows that a total of $71 billion[1] has been invested by 44 HIC governments in global health R&D[2] between 2007-2023, representing 66% of total global investment, with most of the remainder coming from the private sector and from large health-focused philanthropic organisations like the Gates Foundation and Wellcome. Inflation-adjusted annual investment increased gradually between 2007 and 2019, rising by an average of $179 million per year. In 2020, funding surged by $4.5 billion (a 97% increase) in response to the COVID-19 pandemic. This elevated level of investment was sustained into 2021 but has declined in subsequent years – though it remains above pre-pandemic levels.
Which global health areas do HICs governments fund?
Since 2007, using the scope, data, and definitions from the G-FINDER survey, the majority of HIC funding has been directed toward neglected diseases (NDs), which received 63% of total investment. Emerging infectious diseases (EIDs) accounted for 33%, while only a small fraction – 2% – was invested in sexual and reproductive health (SRH). However, these figures are shaped by the gradual expansion of our survey scope over time: NDs have been included since the survey’s inception in 2007; EIDs, beginning with Ebola, were added starting in 2014; and SRH was introduced in 2018. When we limit the analysis to data from 2018 onward, during which, aside from COVID-19, the survey scope has remained relatively stable – the distribution shifts: EIDs represent 53% of HIC funding, NDs 40%, and SRH 4%.
How much of this funding stays within HICs?
Of the $71 billion, 90% ($64 billion) remained in HICs, and most remained within its country of origin: 76% of all HIC funding went to recipients in the same country that provided it. Cross border funding flows between HICs and, especially, funding from the European Commission to EU member states, meant that some countries received more funding than they themselves provided.
Only around 10% of funding flows directly from HIC governments to LMICs.[3] South Africa is by far the largest recipient of this direct funding, receiving 33% of the total, with the next largest recipients – Costa Rica, Brazil, Uganda, Peru and Gambia – all receiving 5% each.
The small share of funding going directly to LMICs reinforces the importance of the role played by Product Development Partnerships (PDPs) and intermediary organisations like the European and Developing Countries Clinical Trials Partnership (EDCTP) and the Coalition for Epidemic Preparedness Innovations (CEPI) which, together, receive 15% of HIC public funding and channel a larger share of it – about 22% of the total – onward to the LMICs in which they work.
Why does most funding remain in HICs?
There are several structural reasons why the majority of global health R&D funding from HICs remains within their borders. Much of this investment is routed through domestic institutions (including universities, government research agencies, and non-profit organisations), that already have the infrastructure, capacity, and track record to receive and manage large-scale public grants. Procurement rules, risk assessments, and reporting standards often favour established entities within donor countries, creating additional barriers to fund disbursement beyond HICs. Eligibility requirements further reinforce this pattern by mandating or strongly encouraging the involvement of researchers or institutions based in the funding country. For example, funding from the US (with partial exceptions from institutions like the NIH Fogarty International Center), the UK, and the European Commission often requires or promotes collaborations between a HIC-based researcher and an LMIC-based partner. While this can facilitate cross-regional exchange, it also structurally embeds funding flows within HIC systems.
In many cases, HIC governments fund research and product development with a global mandate but contract it out to domestic entities. These institutions may collaborate internationally or develop tools for diseases primarily affecting LMICs, but the funding itself remains in the originating country - along with associated jobs, infrastructure investment, and indirect economic benefit. This is particularly the case for basic research, which is the focus of the US National Institutes of Health (NIH), comfortably the largest single funder of global health R&D. Because basic research doesn’t involve testing medicines on patients it can, and typically does, take place at universities and research institutions in the funding nation.
At the same time, some LMIC institutions face challenges in meeting donor requirements for grant-making, limiting their eligibility for direct funding. While there has been sustained investment in this area – including long-standing efforts such as Wellcome’s support to KEMRI since the 1960s and UKCDR's initiatives to strengthen research capacity in LMICs – continued efforts remain critical. Continued investments in LMIC R&D ecosystems is essential to build the capacity, governance, and partnerships needed for a more geographically inclusive and resilient R&D ecosystem.
[1] All our monetary figures are quoted in inflation-adjusted 2024 US dollars, with funding in other currencies converted at average 2024 exchange rates.
[2] By which we mean funding for biopharmaceutical research and development targeting neglected and emerging infectious diseases and women’s health.
[3] We assume that funding with no specified recipient matches the distribution of the funding we do know about, so that the vast majority stay in HICs. See Appendix for details.
What domestic returns do HICs see from investing in global health R&D?
Our research shows that the vast majority – 90% – of funding from HIC governments into global health R&D goes to HIC institutions, although those institutions may then further flow funding or goods onto LMICs. This funding fuels local economies, boosting GDP and creating high-quality research jobs that ripple into other sectors. Far from being an undisputed global public good, government investment in global health R&D catalyses private sector investment and sparks innovation, with patents and scientific breakthroughs multiplying long after the initial funding is gone. Drawing on a thorough review of the academic and other literature, we identified the economic multipliers applicable to each of the countries covered by our G-FINDER survey data to estimate the economic and scientific returns to HICs of two decades of global health R&D investment.
Note: For more details on the individual studies and impact multipliers considered, and why we selected the ones we did, please refer to the Appendix.
Economic activity generated
Investment in research doesn’t stop at the lab – it triggers spending and growth in adjacent sectors, rippling through the economy. Comparing the economic impact of government R&D spending across countries is no easy task. Studies vary widely in their methods, definitions of economic activity, and the types of R&D they assess, making direct comparisons tricky. Still, looking at the high-quality studies most applicable to our data, we found that each dollar invested in R&D returns somewhere between 5.70 and 10.20 to the economy.[1]
Based on these estimates, the $64 billion in R&D funding retained by HICs would be expected to generate around $511 billion in economic activity, within plausible alternative estimates ranging from $365 billion at the low end to as much as $658 billion on the high end. The US alone is projected to generate $387 billion in economic impact from its R&D investments. For ‘Team Europe’ – EU Member States along with the European Commission itself – the figure is almost $57 billion. Much of that return has already been realised during the period from 2007-2023 in which the spending took place, but it also has a long tail. A not-insignificant share of that benefit will continue to accrue over the coming decade, continuing to drive economic growth.
Jobs created
Funding brings higher levels of economic activity that bring more jobs. These jobs, themselves, stimulate more economic activity. While it is comparatively easy to measure the extra jobs created directly in the R&D sector – scientists, technicians, biosecurity officers – it is much more difficult to precisely estimate how many extra jobs the ripples from these new projects create across the whole economy – for delivery drivers, in nearby cafes and for the makers of safety equipment.
The studies underpinning our assessment suggest that every $1 million invested in health R&D generates around 2.7 direct jobs in the research sector, the scientists, lab technicians, and security staff we talked about above. But the ripple effects extend much further. These direct jobs fuel demand across other sectors, from construction and equipment manufacturing and even food services and local retail, ultimately supporting something like ten jobs per $1 million invested across the wider economy. This means, very roughly, that each extra job in R&D leads to a little over two additional jobs in other sectors, either servicing the R&D industry or based on the additional demand from its employees.
Applied to the $64 billion invested globally in health R&D, this equates to approximately 172,000 direct jobs and around 643,000 total jobs. These are often referred to as ‘indirect and induced’ jobs – created through the spending power and economic activity of those directly employed in R&D.
Private sector investment catalysed
Research shows that government spending on R&D doesn’t ‘crowd out’ private investment by soaking up the limited resources available for R&D, but instead ‘crowds it in’ by providing a steady stream of new ideas on which the private sector can capitalise on. Take the example of developing a novel drug for a neglected disease: the high risks, long development timelines, and (often) low prospective returns can deter private investors. In such cases, government grants provide essential funding to move projects through the riskiest early clinical phases. This public investment can then attract additional private capital to complete product development once government funding has supplied proof-of-concept. Public funding can also strengthen innovation networks – whether directly, through public-private partnerships, or indirectly because of researchers moving from academia to pharma companies.
This catalytic effect tends to play out over time. The literature distinguishes between short-term and long-term leverage effects: in the short term (typically within a year) government investment often triggers an immediate stimulus in private R&D spending. The long-term effect, which is harder to study, reflects the cumulative impact usually over a decade or more as sustained public investment continues to draw in private capital. Overall, most estimates suggest that around 60% of the private sector response happens within the first three years.
The effect of public R&D on the private sector varies widely by country, shaped by existing patterns of industry and their links to the government. A cross-country analysis shows the US, UK, Japan, and Germany consistently outperform the OECD average in leveraging public investment to crowd-in private capital. In the US, every public dollar invested returns between $0.85 and $1.25 in private R&D within a year – and eventually up to $7.36. The UK sees similar gains, with £0.73 to £1.03 in the short term and up to £4.02 in the long term. Germany and Japan also show strong results, each exceeding the OECD average of a 1:0.67 multiplier in the short-term and 1:3.72 long-term.
Assuming similar dynamics in global health, we estimate that the $64 billion invested by HIC governments has already catalysed around $62 billion in private R&D, with the potential to grow to $359 billion over the coming decade.
Patents filed
It’s hard to pin down exactly how many patents will result from global health R&D investment. The patenting process is slow, and organisations that are already good at innovating tend to both receive more funding and file more patents, so it can be difficult to determine whether extra funding is the cause or the effect of an organisation’s ability to generate new ideas. Still, two studies using sophisticated statistical methods estimate the amount of funding needed to generate an additional patent at between $2.6 million and $3.8 million. Meanwhile, data from the EU’s Horizon 2020 programme suggests a much higher figure (over €20 million per patent) by dividing its total investment by the number of patents filed so far – likely an underestimate since many projects were still ongoing when the evaluation took place and does not include patents filed by organisation not directly funded through horizon projects. Research suggests that, for every patent induced by targeted R&D funding, as many as three patents may be generated in other technologies by other firms.
Using the $3.2 million estimate (the average from the two non-EU studies), global health R&D investments from HICs could yield around 20,000 patents – driving innovation not just in health but across the wider economy.
[1] At the lower end of the estimated return range is the European Commission’s evaluation of the Horizon 2020 programme, which suggests that every euro invested will generate roughly five euros in economic benefits to EU citizens by 2040. At the higher end is a study by Ciaffi et al., which analysed the macroeconomic effects of public investment in innovation across 15 OECD countries between 1981 and 2017. Using sophisticated ‘Structural Vector Autoregression’ (SVAR) models, the researchers isolated the effects of unexpected government spending shocks and estimated that R&D investment yields a return of 6.26 times the additional investment in the first year, rising to a peak multiplier of 10.33 by the fourth year. Sitting between these two is a study from University College London, which also applied SVAR models to quarterly U.S. data from 1947 to 2017. Focusing on ’mission-oriented‘ innovation spending, the study found that every dollar invested in civil R&D sectors (such as health, energy, and space) can generate up to $7.76 in national output. While these studies do not focus exclusively on biomedical R&D, they offer a useful proxy for its effects on the economy.
Leveraging global health R&D for all: The spillover effects of innovation
The benefits of global health innovations don’t stop at borders, or for the purposes or geographies for which they are originally intended. Many innovations initially developed, trialled, or approved for LMICs have ultimately benefited populations in HICs in various ways: including faster and more cost-effective vaccine development, stronger pandemic preparedness, rapid point-of-care diagnostics to reduce waiting times, and broader access to technologies such as affordable and long-acting contraceptives now also used in high-income settings.
Examples of innovations that delivered dual benefits
This list of 22 innovations (alphabetically ordered) illustrates the broader health, economic and societal benefits of investing in global health R&D, spanning neglected diseases, emerging infectious diseases, and sexual & reproductive health.
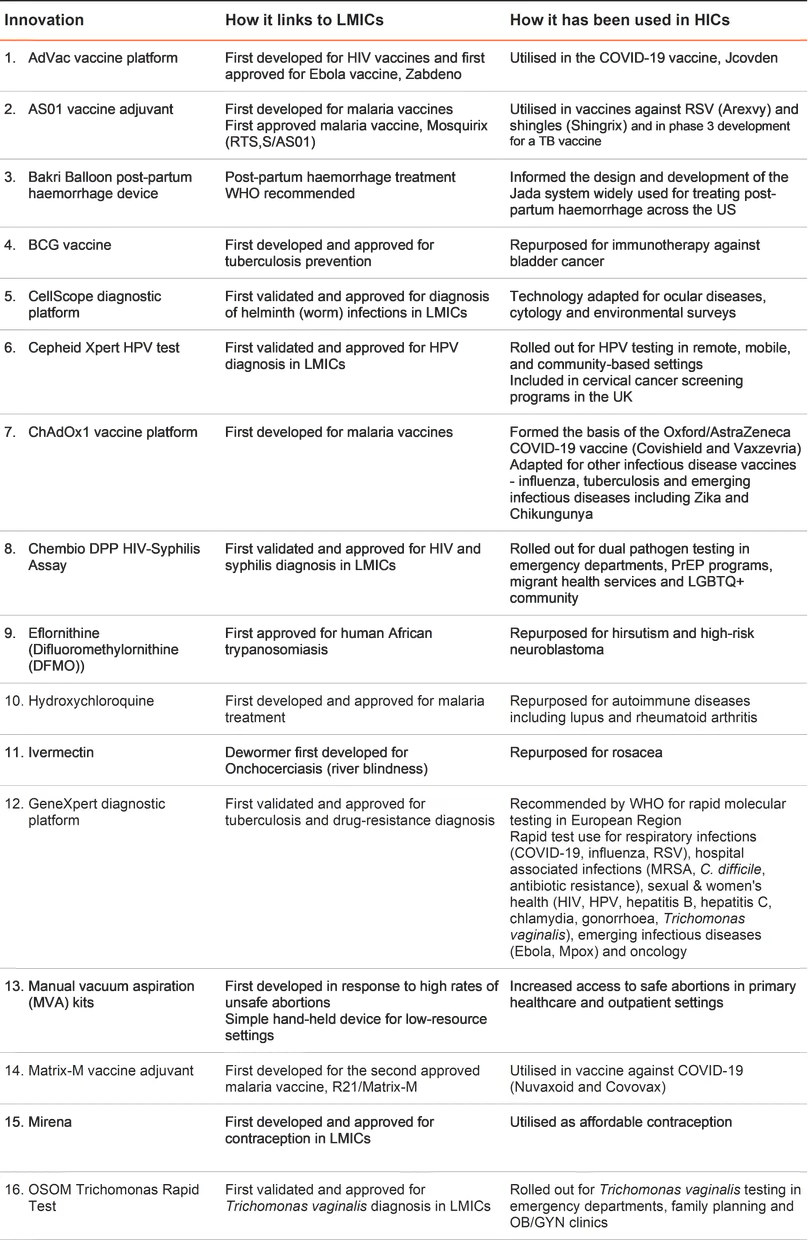
Note: We reviewed academic and grey literature using specific search terms and inclusion criteria to map innovations with these dual benefits and leveraged our in-house expertise. Our inclusion criteria are defined by the G-FINDER survey scope, which has restrictions around certain products. There are more products than those we have listed that have dual benefits, but these do not feature here due to our scope, for example the AS04 vaccine adjuvant developed for a Hepatitis B vaccine is excluded because Hepatitis B vaccines are not in the G-FINDER scope. See the Appendix for more details.
Case studies
- CASE STUDY ONE
Beyond disease-specific vaccines: the economic power of vaccine adjuvants
Innovations first developed to tackle malaria in LMICs are now delivering health and economic returns across HICs. Adjuvants – compounds that boost vaccine effectiveness – provide a good example of this. AS01 and Matrix-M were originally designed for malaria vaccines like RTS,S and R21 to stimulate strong, cell-mediated immune responses. With 24 million doses of malaria vaccines distributed across 20 African nations already, the AS01 and Matrix-M adjuvants are now being harnessed to boost the effects of blockbuster vaccines in HICs.
AS01 developed for RTS,S is now key to Arexvy, the first approved vaccine against respiratory syncytial virus (RSV), which has been administered to over 9 million adults over 60 years of age in the US since 2023, generating over $1.5 billion in revenue in its first six months. AS01 is also used in Shingrix, a shingles vaccine distributed in over 50 countries since its approval in 2017, is credited with reducing severe herpes zoster cases and had a market valued at $4 billion in 2024.
The other vaccine adjuvant, Matrix-M, was initially developed for an influenza vaccine but was first clinically validated in combination with malaria-targeting R21 vaccine. It was then later integrated into Novavax’s COVID-19 vaccines (Nuvaxovid and Covovax), which are approved in over 40 markets, mostly in HICs. These COVID-19 vaccines generated nearly $3 billion in revenue between 2022 and 2023, and would not have been possible without Matrix-M.
The potential of these two adjuvants is far from exhausted. AS01 is also being investigated in a new tuberculosis vaccine that could reduce the burden and transmission, including from multidrug-resistant forms, seen in LMICs and, increasingly, in HICs as well. One of the most advanced TB vaccine candidates, M72/AS01, is running ahead of schedule in its Phase III trials and could be the first TB vaccine approved since the introduction of BCG over a century ago. Modelling studies suggest M72/AS01 vaccination could avert 40% more TB cases and be seven times more cost-effective than BCG re-vaccination.
- CASE STUDY TWO
Contraception & women’s health: Bridging gaps to support global contraceptive choice
Access to contraception is a human right, as well as a cornerstone of reproductive autonomy, women’s health and gender equality. Despite FDA-approval of the first oral contraceptive in 1960, contraceptive innovation remains essential to meet diverse needs and improve uptake and access. Since the Dalkon Shield IUD scandal in the 1970s, industry investment has declined due to liability concerns and high insurance rates, meaning most contraceptive innovations from the last fifty years were developed by publicly funded nonprofit organisations. One example is the Population Council, which has led contraceptive R&D with the needs of populations in LMICs in mind, but whose impact is global. Funded by public agencies (e.g. USAID and the US NIH), philanthropy and multilateral organisations (e.g. UNFPA and the WHO), it has developed several major contraceptive innovations including the copper IUD, Paragard; the first long-acting, reversible contraceptive implant, Norplant, that was later improved as the Jadelle implant; the vaginal ring, Annovera; and the hormonal intra-uterine system, Mirena.
In the 1970’s, the Population Council pioneered hormone-releasing IUDs, focusing on levonorgestrel and sought industry partners to support manufacturing and global distribution. It licensed the patent to Leiras Oy, later acquired by Schering (1996) and then Bayer (2006), which led to the launch of the Mirena IUD. Through the International Contraceptive Access Foundation established with Bayer AG in 2003, a donated version of Mirena (LNG IUS) is available in LMICs, but Mirena is now used all over the world, including in HICs. Hormonal IUDs now represent 74% of the market share for IUDs globally. In the USA, 11% of women of reproductive age use IUDs (with 76.5% being hormonal IUDs). In Nordic countries, hormonal IUDs are used by 10-15% of reproductive aged women, while user rates in France are 8%. Beyond contraception, Mirena IUD is also recommended for heavy menstrual bleeding and is used off-label for endometriosis related pelvic pain and menstrual bleeding.
Although focused on LMICs, the Population Council’s contraceptive innovation has revolutionised the field globally, bridging industry gaps and expanding cost effective contraceptive solutions to both LMICs and HICs.
- CASE STUDY THREE
Lessons in acceleration: how past innovations shaped the COVID-19 response
The COVID-19 pandemic triggered a critical reassessment of established R&D methods and traditional, lengthy development timelines. As a result, existing innovations and platform technologies initially investigated for other infectious diseases came to the forefront to speed up development and approval. This enabled the first COVID-19 vaccines to be available to the public less than a year after the virus was sequenced, a process which typically takes 5-10 years, reducing by half the usual $800 million price tag for vaccine development.
The Oxford/AstraZeneca vaccine is a case in point. It was built on the ChAdOx1 adenoviral vector vaccine platform, originally developed for malaria, and later adapted for influenza, TB, Zika and Chikungunya. It formed the backbone of the Oxford/AstraZeneca COVID-19 vaccine, which required no ultra-cold-chain and was more affordable than rival mRNA options, helping it save over 6 million lives during its first year. Its development was driven by public funding – 26% from the UK, 27% from overseas (mostly the EU) – and philanthropic organisations (24%).
SORMAS (Surveillance, Outbreak Response Management and Analysis System), open-source software developed during the West African Ebola outbreak in 2014, was scaled up rapidly during the pandemic to support real-time outbreak tracking not only in Africa but also in parts of Asia and Europe.
Remdesivir, initially tested for hepatitis C and Ebola, became one of the first approved COVID-19 treatments in Australia and the EU by mid-2020, and in the US by November that year. Among high-risk patients it cut mortality by 20 per 1,000 and reduced hospitalisation or death by 87%. In Italy, its use among high-risk, non-hospitalised patients was estimated to have saved €50.8 million and prevented up to 1,100 deaths. Another model projected €431 million in savings and over 17,000 ICU admissions avoided for Italian patients on low-flow oxygen.
GeneXpert, a cartridge-based molecular diagnostic platform, was originally developed for TB and rifampicin resistance detection. This platform revolutionised TB diagnostics by delivering results in under two hours with high sensitivity and specificity, even in decentralised or low-resource settings. Between 1992-2020, the US developer Cepheid received over $250 million in public funding (mostly from the US) to create the platform and adapt it to numerous other infectious diseases, including COVID-19. The platform reduced time to diagnosis, resulting in a significant decrease in infection-control resource consumption with savings calculated to be over $650,000 in non-reusable PPE in two US-based hospitals alone.
The above examples demonstrate a direct repurposing of existing innovations for COVID-19, but R&D enablers and regulatory processes also benefited from previous investment and R&D for LMIC-focused innovations. The Ebola vaccine, ERVEBO, was the first approved vaccine to leverage the rVSV platform. It’s emergency approval for Ebola demonstrated that non-traditional vaccine platforms could be safe and effective. It set a regulatory precedent, providing a template for the emergency use frameworks that were crucial during the COVID-19 response. The rapid rollout of ERVEBO also informed deployment strategies for COVID-19 vaccines, particularly for cold-chain logistics, community engagement and safety monitoring.
- CASE STUDY FOUR
Everything old is new again: fast-tracking approvals through repurposing
Repurposing biomedical innovations is a cost-effective strategy that accelerates development by leveraging existing safety and toxicity data. It reduces R&D costs, shortens approval timelines, especially during public health emergencies, and salvages value from previously unsuccessful candidates, minimising waste and maximising return on investment.
One notable example is eflornithine. Initially investigated and then discarded as a potential cancer treatment in the 1970s, eflornithine was picked up and developed for the treatment of human African trypanosomiasis (sleeping sickness), for which it was approved by the FDA in 1990. Since then, eflornithine has been successfully repurposed to treat female hirsutism and high-risk neuroblastoma, with FDA approvals in 2000 and 2023, respectively. Used topically for hirsutism and orally for prevention of neuroblastoma relapse, it significantly improves outcomes – reducing unwanted hair by 58% and achieving a four-year event-free survival rate of 84% in children with neuroblastoma. Following Australian approval for neuroblastoma in 2025, global expansion efforts are underway, led by Norgine and US WorldMeds.
Similarly, BCG, originally developed and approved nearly a century ago for TB prevention, remains one of the most widely used vaccines today. BCG has been repurposed as the first approved cancer immunotherapy for non-muscle invasive bladder cancer (NMIBC). Now widely used in HICs, BCG therapy significantly lowers recurrence and progression rates following standard bladder cancer surgery, while helping curb long-term treatment costs, estimated at €3,900 per patient over five years. With bladder cancer cases and deaths projected to rise sharply by 2040, BCG has become a vital and cost-effective part of HIC health systems’ responses.
This pattern is also reflected in hydroxychloroquine, originally designed and approved for the treatment of malaria. Over time, it has been repurposed and approved for treating autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, where it now serves as a first-line therapy for SLE in the US. With millions affected globally by both conditions, it is widely used in HICs including Australia, the US, Canada, UK and the EU. In 2022, it was among the top 150 most prescribed drugs in the US, with over five million prescriptions.
Implications for the global health R&D ecosystem of the future
The current global health R&D system has delivered extraordinary life-saving innovations, health, economic and scientific benefits globally. HICs have seen significant benefits which they can continue to reap given the concentration of infrastructure and expertise developed over many years in these countries – if governments sustain their investment and the private sector is stimulated to co-invest.
Whilst the concentration of R&D investment in HICs has delivered significant returns, this model is reaching its limits. The next phase of global health R&D requires a deliberate transition toward more distributed capacity, not despite the economic benefits to HICs, but to sustain and amplify them. It is in the interests of both HIC and LMIC governments to strengthen this ecosystem further.
Empowering LMICs through investment in local and regional R&D
LMIC governments have a pivotal role to play in shaping a more resilient and equitable global R&D ecosystem. The evidence is clear: investing in health R&D delivers not only better health outcomes but also catalyses economic growth, industrial development, and scientific advancement. To fully realise these benefits, LMICs must prioritise investment in infrastructure and capacity building including:
- Building and maintaining research infrastructure and biomanufacturing capacity to enable local and regional product development and scale-up.
- Investing in human capital through sustained support for STEM education, clinical trial expertise, and leadership in scientific research.
- Strengthening regulatory systems and ethical review mechanisms to ensure that research is safe, efficient, and globally interoperable.
- Developing innovation-friendly policies and governance structures that support knowledge transfer, data stewardship, public-private cooperation, public procurement, and local ownership of research agendas.
There is growing recognition that we need to rethink how global health R&D is financed. To fully unlock the potential of LMICs in global health R&D, financing models must evolve to be more responsive to today’s realities. Traditional funding approaches, largely driven by public and philanthropic institutions in HICs, have underwritten many successes, but often concentrate resources in a limited number of geographies and institutions, limiting opportunities for local leadership.
A more sustainable and inclusive R&D system will require a rebalancing of risk, responsibility, and return. This means:
- Expanding matched co-funding mechanisms that catalyse domestic investment from LMIC governments.
- Deploying blended finance instruments that use public or philanthropic capital to crowd in private investment.
- Channelling more funding directly to LMIC institutions.
- Reforming existing funding flows to prioritise long-term capacity-building, not just product delivery.
PDPs: critical to strengthen the future R&D ecosystem
PDPs are among the most effective vehicles for delivering affordable, context-appropriate technologies. Data also show that a significant share of PDP funding (78%) remains in HICs. This highlights an opportunity to better align these partnerships with long-term capacity goals in LMICs. To do so, PDPs need not only increased funding, but also the flexibility to invest in strengthening local research infrastructure, workforce development, and regulatory systems. Currently, many PDPs face constraints due to narrowly scoped funding, limiting their ability to contribute to broader ecosystem-building, a challenge often described as the ‘unfunded mandate’ of PDPs. Funders should seize the opportunity to fund these ‘unfunded mandates’: the cross-cutting functions that fall outside traditional product budgets but are vital for building local R&D ecosystems. This includes support for LMIC-led trials, technology transfer, regulatory harmonisation, and regional leadership. By empowering PDPs to take on these roles, donors can help shift the model from one-way technology transfer to true partnership anchored in resilient, locally driven innovation systems.
These approaches are not about shifting funding away from high-performing institutions in HICs. They are about broadening the base, designing financing models that build strength across more geographies, ensure the durability of R&D pipelines, and create greater shared value.
For donors, now is the time for strategic, forward-looking investments which can help build a more distributed and resilient R&D ecosystem: one that protects hard-won gains, accelerates local innovation, and ensures that the benefits of global health research are more equitably shared.
Call to action
The evidence is clear: investing in global health R&D drives growth in countries with the infrastructure, institutions, and expertise to absorb and leverage it.
Protect and expand current investments
HICs receive substantial economic and security returns from global health research and development: each dollar invested creates high-technology employment, advances adaptable platform technologies that can be redeployed quickly in crises, fosters university-industry collaboration, and generates significant downstream market activity. The ChAdOx1 vaccine backbone, GeneXpert diagnostics, and the SORMAS surveillance system, initially developed for lower-resource settings, proved critical during recent emergencies and illustrate this value.
Reducing investment in global health R&D would weaken a proven engine of growth and preparedness at the very moment when antimicrobial resistance, climate-related disease patterns, and demographic change are increasing demand for innovative countermeasures.
Maintaining and, where possible, expanding HIC funding is therefore essential for national competitiveness and global health security.
Invest in LMIC-led R&D to build global resilience
Greater investment in LMIC-led R&D that strengthens local research capacity, infrastructure, and regulatory systems unlocks innovation, creates jobs, and accelerates access to life-saving tools. It also helps detect and contain emerging threats before they spread. In a deeply interconnected world, investing in LMIC-led R&D is a critical strategy for building shared resilience. Empowering PDPs and intermediaries to build capacity in LMICs can help shift the model from one-way technology transfer to true partnership anchored in resilient, locally driven innovation systems.
Reap the rewards well into the future
Reducing investments in global health R&D now would undermine present and future health and economic gains, for LMICs as well as HICs. To sustain these benefits into the future, donors must maintain their investment and adopt partnership models and innovative financing approaches that amplify impact. With smart investments and strong partnerships, we can continue building a resilient, innovative, and equitable global health future that delivers benefits domestically and globally.

PDF of the report
Download a PDF of the full report

Video of the key messages
View a quick summary of the key statistics and messages

The Impact of Global Health R&D Hub
Visit our Hub for a series of reports and insights assessing the impact of global health R&D
References
Access to Medicine Foundation. (2022, November 15). A holistic approach to expanding access to contraceptive products. https://accesstomedicinefoundation.org/resource/bayer-applies-a-variety-of-methods-to-reach-different-population-segments-and-provide-access
Aderinto, N., Olatunji, G., Kokori, E., Sikirullahi, S., Aboje, J. E., & Ojabo, R. E. (2024). A perspective on Oxford’s R21/Matrix-MTM malaria vaccine and the future of global eradication efforts. Malaria Journal, 23(1), 16. https://doi.org/10.1186/s12936-024-04846-w
Amstutz, A., Speich, B., Mentré, F., Rueegg, C. S., Belhadi, D., Assoumou, L., Burdet, C., Murthy, S., Dodd, L. E., Wang, Y., Tikkinen, K. A. O., Ader, F., Hites, M., Bouscambert, M., Trabaud, M. A., Fralick, M., Lee, T. C., Pinto, R., Barratt-Due, A., … Briel, M. (2023). Effects of remdesivir in patients hospitalised with COVID-19: A systematic review and individual patient data meta-analysis of randomised controlled trials. The Lancet. Respiratory Medicine, 11(5), 453–464. https://doi.org/10.1016/S2213-2600(22)00528-8
Auro Vaccines. (2025). VesiculoVaxTM Prophylactic Vaccines. https://aurovaccines.com/pipeline/vesiculovax-prophylactic-vaccines/
Azoulay, P., Zivin, J. S. G., Li, D., & Sampat, B. N. (2015). Public R&D Investments and Private-sector Patenting: Evidence from NIH Funding Rules.
Barańska-Rybak, W., & Kowalska-Olędzka, E. (2019). New indications for topical ivermectin 1% cream: A case series study. Postepy Dermatologii I Alergologii, 36(1), 58–62. https://doi.org/10.5114/ada.2019.82825
Barber, M. R., Pierre, Y. S., Hanly, J. G., Urowitz, M. B., Gordon, C., Bae, S.-C., Romero-Diaz, J., Sanchez-Guerrero, J., Bernatsky, S., Wallace, D. J., Isenberg, D. A., Rahman, A., Merrill, J. T., Fortin, P. R., Gladman, D. D., Bruce, I. N., Petri, M., Ginzler, E. M., Dooley, M. A., … Clarke, A. E. (2021). 1107 Economic evaluation of hydroxychloroquine use in an international inception cohort. Abstracts, A33.2-A34. https://doi.org/10.1136/lupus-2021-lupus21century.50
Breakthrough Energy. (2020). Impacts of Federal R&D Investment on the US Economy.
Caya, C., Maheu-Giroux, M., Xia, Y., Serhir, B., Morin, V., Libman, M., Corsini, R., Goldfarb, D. M., Wong, T., Singh, A. E., & Yansouni, C. P. (2022). Stopping syphilis transmission in Arctic communities through rapid diagnostic testing: The STAR study protocol. PloS One, 17(9), e0273713. https://doi.org/10.1371/journal.pone.0273713
CellScope UC Berkley. (2025). CellScope Applications. https://cellscope.berkeley.edu/applications/
Cepheid. (2025). Cepheid Global Product Catalog. https://www.cepheid.com/global-products.html
Chandler, R., Montenegro, N., Llorach, C., Aguirre, L. N., Germain, S., Kuriyakose, S. O., Lambert, A., Descamps, D., Olivier, A., & Hulstrøm, V. (2024). Immunogenicity, Reactogenicity, and Safety of AS01E-adjuvanted RSV Prefusion F Protein-based Candidate Vaccine (RSVPreF3 OA) When Co-administered With a Seasonal Quadrivalent Influenza Vaccine in Older Adults: Results of a Phase 3, Open-Label, Randomized Controlled Trial. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, ciad786. https://doi.org/10.1093/cid/ciad786
Ciaffi, G., Deleidi, M., & Mazzucato, M. (2024). Measuring the macroeconomic responses to public investment in innovation: Evidence from OECD countries. Industrial and Corporate Change, 33(2), 363–382. https://doi.org/10.1093/icc/dtae005
ClinCalc DrugStats Database. (2025). Hydroxychloroquine—Drug Usage Statistics. https://clincalc.com/DrugStats/Drugs/Hydroxychloroquine
Curran, D., Patterson, B., Varghese, L., Van Oorschot, D., Buck, P., Carrico, J., Hicks, K., Lee, B., & Yawn, B. (2018). Cost-effectiveness of an Adjuvanted Recombinant Zoster Vaccine in older adults in the United States. Vaccine, 36(33), 5037–5045. https://doi.org/10.1016/j.vaccine.2018.07.005
Deleidi, M., Mazzucato, M., De Lipsis, V., & ryan-collins, J. (2020). The macroeconomic impact of government innovation policies: A quantitative assessment. UCL Institute for Innovation and Public Purpose, Policy Report working paper series (IIPP WP 2019-06). https://www.ucl.ac.uk/bartlett/public-purpose/wp2019-06
Didierlaurent, A. M., Laupèze, B., Di Pasquale, A., Hergli, N., Collignon, C., & Garçon, N. (2017). Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Review of Vaccines, 16(1), 55–63. https://doi.org/10.1080/14760584.2016.1213632
Diemert, A., Ortmeyer, G., Hollwitz, B., Lotz, M., Somville, T., Glosemeyer, P., Diehl, W., & Hecher, K. (2012). The combination of intrauterine balloon tamponade and the B-Lynch procedure for the treatment of severe postpartum hemorrhage. American Journal of Obstetrics and Gynecology, 206(1), 65.e1-4. https://doi.org/10.1016/j.ajog.2011.07.041
Dima, A., Jurcut, C., & Arnaud, L. (2021). Hydroxychloroquine in systemic and autoimmune diseases: Where are we now? Joint Bone Spine, 88(3), 105143. https://doi.org/10.1016/j.jbspin.2021.105143
Dos Reis Neto, E. T., Kakehasi, A. M., de Medeiros Pinheiro, M., Ferreira, G. A., Marques, C. D. L., da Mota, L. M. H., Dos Santos Paiva, E., Pileggi, G. C. S., Sato, E. I., Reis, A. P. M. G., Xavier, R. M., & Provenza, J. R. (2020). Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Advances in Rheumatology (London, England), 60(1), 32. https://doi.org/10.1186/s42358-020-00134-8
European Commission. (2024). Ex post evaluation of Horizon 2020, the EU framework programme for research and innovation. Report from the Commission to the European Parliament and the Council.
European Medicines Agency (EMA). (2025, January 24). Ervebo. https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo
Falsey, A. R., Sobieszczyk, M. E., Hirsch, I., Sproule, S., Robb, M. L., Corey, L., Neuzil, K. M., Hahn, W., Hunt, J., Mulligan, M. J., McEvoy, C., DeJesus, E., Hassman, M., Little, S. J., Pahud, B. A., Durbin, A., Pickrell, P., Daar, E. S., Bush, L., … AstraZeneca AZD1222 Clinical Study Group. (2021). Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. The New England Journal of Medicine, 385(25), 2348–2360. https://doi.org/10.1056/NEJMoa2105290
Fernàndez-López, L., Reyes-Urueña, J., Egea, L., Chernyshev, A., Upmace, I., Ćosić, M., Mejías, W., González, V., Blondeel, K., Thwin, S. S., Gios, L., Mirandola, M., Peeling, R., Kiarie, J., Casabona, J., Toskin, I., & ProSPeRo group. (2024). A clinical utility evaluation of dual HIV/Syphilis point-of-care tests in non-clinical settings for screening for HIV and syphilis in men who have sex with men. BMC Infectious Diseases, 24(Suppl 1), 264. https://doi.org/10.1186/s12879-024-09017-5
Folegatti, P. M., Ewer, K. J., Aley, P. K., Angus, B., Becker, S., Belij-Rammerstorfer, S., Bellamy, D., Bibi, S., Bittaye, M., Clutterbuck, E. A., Dold, C., Faust, S. N., Finn, A., Flaxman, A. L., Hallis, B., Heath, P., Jenkin, D., Lazarus, R., Makinson, R., … Oxford COVID Vaccine Trial Group. (2020). Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet (London, England), 396(10249), 467–478. https://doi.org/10.1016/S0140-6736(20)31604-4
Fraser of Allander Institute. University of Strathclyde. (2021). The contribution of medical research funding by charities to the UK economy.
Gilead Sciences. (2020, July 3). European Commission Grants Conditional Marketing Authorization for Gilead’s Veklury® (remdesivir) for the Treatment of COVID-19. https://www.gilead.com/news/news-details/2020/european-commission-grants-conditional-marketing-authorization-for-gileads-veklury-remdesivir-for-the-treatment-of-covid-19
Gliddon, H. D., Peeling, R. W., Kamb, M. L., Toskin, I., Wi, T. E., & Taylor, M. M. (2017). A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sexually Transmitted Infections, 93(S4), S3–S15. https://doi.org/10.1136/sextrans-2016-053069
Gotham, D., McKenna, L., Deborggraeve, S., Madoori, S., & Branigan, D. (2021). Public investments in the development of GeneXpert molecular diagnostic technology. PLoS ONE, 16(8), e0256883. https://doi.org/10.1371/journal.pone.0256883
Grönvall, M., Tikkanen, M., Tallberg, E., Paavonen, J., & Stefanovic, V. (2013). Use of Bakri balloon tamponade in the treatment of postpartum hemorrhage: A series of 50 cases from a tertiary teaching hospital. Acta Obstetricia Et Gynecologica Scandinavica, 92(4), 433–438. https://doi.org/10.1111/j.1600-0412.2012.01531.x
Hawash, Y., Jaafer, N., & Alpakistany, T. (2021). Ease of use and validity testing of a point-of-care fast test for parasitic vaginosis self-diagnosis. Tropical Biomedicine, 38(4), 491–498. https://doi.org/10.47665/tb.38.4.094
Hsieh, Y.-H., Lewis, M. K., Viertel, V. G., Myer, D., Rothman, R. E., & Gaydos, C. A. (2020). Performance evaluation and acceptability of point-of-care Trichomonas vaginalis testing in adult female emergency department patients. International Journal of STD & AIDS, 31(14), 1364–1372. https://doi.org/10.1177/0956462420956532
Jiang, S., & Redelman-Sidi, G. (2022). BCG in Bladder Cancer Immunotherapy. Cancers, 14(13), 3073. https://doi.org/10.3390/cancers14133073
Johns Hopkins University. (2022). The Impact of Rotavirus Vaccination. https://publichealth.jhu.edu/sites/default/files/2024-02/rota-brief7-vaccineimpact2022ax.pdf
Kakinuma, T., Kakinuma, K., Sakamoto, Y., Kawarai, Y., Saito, K., Ihara, M., Matsuda, Y., Sato, I., Ohwada, M., Yanagida, K., & Tanaka, H. (2020). Safety and efficacy of manual vacuum suction compared with conventional dilatation and sharp curettage and electric vacuum aspiration in surgical treatment of miscarriage: A randomized controlled trial. BMC Pregnancy and Childbirth, 20(1), 695. https://doi.org/10.1186/s12884-020-03362-4
Kennedy, C. E., Yeh, P. T., Gaffield, M. L., Brady, M., & Narasimhan, M. (2019). Self-administration of injectable contraception: A systematic review and meta-analysis. BMJ Global Health, 4(2), e001350. https://doi.org/10.1136/bmjgh-2018-001350
KPMG. (2018). Economic Impact of Medical Research in Australia. A report prepared for the Association of Australian Medical Research Institutes.
Lalonde, A. & International Federation of Gynecology and Obstetrics. (2012). Prevention and treatment of postpartum hemorrhage in low-resource settings. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics, 117(2), 108–118. https://doi.org/10.1016/j.ijgo.2012.03.001
Ledford, H. (2021). Malaria vaccine wows and seeds COVID-19 vaccine effort. Nature Biotechnology, 39(7), 786–787. https://doi.org/10.1038/s41587-021-00983-8
LoGiudice, N., Le, L., Abuan, I., Leizorek, Y., & Roberts, S. C. (2018). Alpha-Difluoromethylornithine, an Irreversible Inhibitor of Polyamine Biosynthesis, as a Therapeutic Strategy against Hyperproliferative and Infectious Diseases. Medical Sciences, 6(1), 12. https://doi.org/10.3390/medsci6010012
Louw, C., Paffenholz, R., Verset, C., & Krause, G. (2022). Global Good Open Source Software Development in Response to the COVID-19 Pandemic—Perspectives from SORMAS Implementation in Europe. Studies in Health Technology and Informatics, 294, 669–673. https://doi.org/10.3233/SHTI220553
Maliszewski, K. N., Hsieh, Y.-H., Curbeam, D., Rizkallah, A., Perez, D. A., Dashler, G., Ricketts, E. P., Rompalo, A. M., Gaydos, C. A., Manabe, Y. C., Melendez, J., & Rothman, R. E. (2024). An Evaluation of the Performance, Patient Acceptability, and Feasibility of a Point-of-Care HIV-Syphilis Assay in an Urban Emergency Department. Sexually Transmitted Diseases, 51(10), 648–653. https://doi.org/10.1097/OLQ.0000000000001995
Maliszewski, K. N., Hsieh, Y.-H., Myer, D., Perez, D. A., Gaydos, C. A., Manabe, Y. C., Ricketts, E., & Rothman, R. E. (2021). 1028. Performance and Patient Acceptability Evaluation of the Chembio DPP® HIV-Syphilis Assay in an Emergency Department. Open Forum Infectious Diseases, 8(Suppl 1), S605. https://doi.org/10.1093/ofid/ofab466.1222
Middleton, B. F., Danchin, M., Fathima, P., Bines, J. E., Macartney, K., & Snelling, T. L. (2023). Review of the health impact of the oral rotavirus vaccine program in children under 5 years in Australia: 2006 - 2021. Vaccine, 41(3), 636–648. https://doi.org/10.1016/j.vaccine.2022.12.008
Mousseau, S., Lapointe, A., & Gravel, J. (2018). Diagnosing acute otitis media using a smartphone otoscope; a randomized controlled trial. The American Journal of Emergency Medicine, 36(10), 1796–1801. https://doi.org/10.1016/j.ajem.2018.01.093
National Centre for Universities and Business. (2024). Unlocking growth: The impact of public R&D spending on private sector investment in the UK. Research Series.
Neuroblastoma Asutralia. (2022, September 13). DFMO Updates. Neuroblastoma Australia. https://www.neuroblastoma.org.au/dfmoupdate
NHS England. (2024). Cervical screening: Acceptable HPV tests. https://www.gov.uk/government/publications/cervical-screening-acceptable-hpv-tests/cervical-screening-acceptable-hpv-tests
Novavax. (2025). Matrix-M Adjuvant—Enhancing immune response. https://www.novavax.com/science-technology/matrix-m-adjuvant
Organon. (2025). The JADA® System. https://organonpro.com/en-us/product/the-jada-system/the-jada-system/
Parkins, K. (2021). J&J’s single-shot Covid-19 vaccine: The journey to approval. https://www.clinicaltrialsarena.com/features/jj-single-shot-vaccine-covid-19-approval-timeline/?cf-view
PATH. (2015, September 23). PATH welcomes groundbreaking regulatory approval for contraceptive self-injection. https://www.path.org/our-impact/media-center/path-welcomes-groundbreaking-regulatory-approval-for-contraceptive-self-injection/
Pritchard, C., Kutikova, L., Pitman, R., Lai, K. Z. H., Beyhaghi, H., Gibbons, Ii., Erbe, A., Živković-Gojović, M., Cosgrove, C., Sculpher, M., & Salisbury, D. (2025). Cost-Effectiveness of Introducing Nuvaxovid to COVID-19 Vaccination in the United Kingdom: A Dynamic Transmission Model. Vaccines, 13(2), 187. https://doi.org/10.3390/vaccines13020187
Public Health England. (2017, January 25). NHS Cervical Screening Programme approves new HPV tests and issues guidance for laboratories. https://phescreening.blog.gov.uk/2017/01/25/nhs-cervical-screening-programme-approves-new-hpv-tests-and-issues-guidance-for-laboratories/
Reimer, J. M., Karlsson, K. H., Lövgren-Bengtsson, K., Magnusson, S. E., Fuentes, A., & Stertman, L. (2012). Matrix-MTM adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PloS One, 7(7), e41451. https://doi.org/10.1371/journal.pone.0041451
Roman, F., Burny, W., Ceregido, M. A., Laupèze, B., Temmerman, S. T., Warter, L., & Coccia, M. (2024). Adjuvant system AS01: From mode of action to effective vaccines. Expert Review of Vaccines, 23(1), 715–729. https://doi.org/10.1080/14760584.2024.2382725
Saidu, R., Kuhn, L., Tergas, A., Boa, R., Moodley, J., Svanholm-Barrie, C., Persing, D., Campbell, S., Tsai, W.-Y., Wright, T. C., & Denny, L. (2020). Performance of Xpert HPV on Self-collected Vaginal Samples for Cervical Cancer Screening Among Women in South Africa. Journal of Lower Genital Tract Disease, 25(1), 15–21. https://doi.org/10.1097/LGT.0000000000000575
Schmit, N., Topazian, H. M., Natama, H. M., Bellamy, D., Traoré, O., Somé, M. A., Rouamba, T., Tahita, M. C., Bonko, M. D. A., Sourabié, A., Sorgho, H., Stockdale, L., Provstgaard-Morys, S., Aboagye, J., Woods, D., Rapi, K., Datoo, M. S., Lopez, F. R., Charles, G. D., … Winskill, P. (2024). The public health impact and cost-effectiveness of the R21/Matrix-M malaria vaccine: A mathematical modelling study. The Lancet. Infectious Diseases, 24(5), 465–475. https://doi.org/10.1016/S1473-3099(23)00816-2
SH:24. (2025). Contraceptive Injection. https://sh24.org.uk/contraception/injection/using-injection
Shah, I. H., Kollydas, K., Lee, P. Y., Malki, I., & Chu, C. (2024). Does R&D investment drive employment growth? Empirical evidence at industry level from Japan. International Journal of Finance & Economics, 29(1), 102–118. https://doi.org/10.1002/ijfe.2677
Sholler, G. L. S., Ferguson, W., Bergendahl, G., Bond, J. P., Neville, K., Eslin, D., Brown, V., Roberts, W., Wada, R. K., Oesterheld, J., Mitchell, D., Foley, J., Parikh, N. S., Eshun, F., Zage, P., Rawwas, J., Sencer, S., Pankiewicz, D., Quinn, M., … Kraveka, J. M. (2018). Maintenance DFMO Increases Survival in High Risk Neuroblastoma. Scientific Reports, 8(1), 14445. https://doi.org/10.1038/s41598-018-32659-w
SORMAS Foundation. (2025). SORMAS Overview. https://sormas.org/sormas/overview/
Spencer, B. A., McBride, R. B., Hershman, D. L., Buono, D., Herr, H. W., Benson, M. C., Gupta-Mohile, S., & Neugut, A. I. (2013). Adjuvant intravesical bacillus calmette-guérin therapy and survival among elderly patients with non-muscle-invasive bladder cancer. Journal of Oncology Practice, 9(2), 92–98. https://doi.org/10.1200/JOP.2011.000480
Stein, L., Kircik, L., Fowler, J., Tan, J., Draelos, Z., Fleischer, A., Appell, M., Steinhoff, M., Lynde, C., Liu, H., & Jacovella, J. (2014). Efficacy and safety of ivermectin 1% cream in treatment of papulopustular rosacea: Results of two randomized, double-blind, vehicle-controlled pivotal studies. Journal of Drugs in Dermatology: JDD, 13(3), 316–323.
Stertman, L., Palm, A.-K. E., Zarnegar, B., Carow, B., Lunderius Andersson, C., Magnusson, S. E., Carnrot, C., Shinde, V., Smith, G., Glenn, G., Fries, L., & Lövgren Bengtsson, K. (2023). The Matrix-MTM adjuvant: A critical component of vaccines for the 21st century. Human Vaccines & Immunotherapeutics, 19(1), 2189885. https://doi.org/10.1080/21645515.2023.2189885
Stylianou, V. V., Bertram, K. M., Vo, V. A., Dunn, E. B., Baharlou, H., Terre, D. J., Elhindi, J., Elder, E., French, J., Meybodi, F., Temmerman, S. T., Didierlaurent, A. M., Coccia, M., Sandgren, K. J., & Cunningham, A. L. (2024). Innate immune cell activation by adjuvant AS01 in human lymph node explants is age independent. The Journal of Clinical Investigation, 134(22), e174144. https://doi.org/10.1172/JCI174144
Sussex, J., Feng, Y., Mestre-Ferrandiz, J., Pistollato, M., Hafner, M., Burridge, P., & Grant, J. (2016). Quantifying the economic impact of government and charity funding of medical research on private research and development funding in the United Kingdom. BMC Medicine, 14(1), 32. https://doi.org/10.1186/s12916-016-0564-z
Tabakovic, H., & Wollmann, T. G. (2019). The impact of money on science: Evidence from unexpected NCAA football outcomes. Journal of Public Economics, 178, 104066. https://doi.org/10.1016/j.jpubeco.2019.104066
The Jenner Institute. (2025). Development of the ChAdOx vaccine platform. https://www.jenner.ac.uk/about/the-oxford-astrazeneca-covid-19-vaccine/ChAdOx-platform
The Royal Australian College of General Practitioners. (2024, June 21). Mirena lifespan extended to eight years. https://www1.racgp.org.au/newsgp/clinical/mirena-lifespan-extended-to-eight-years
Therapeutic Goods Administration (TGA). (2020, July 10). Australia’s first COVID treatment approved. https://www.tga.gov.au/news/media-releases/australias-first-covid-treatment-approved
Tötterman, M., Jukarainen, S., Sinkkonen, S. T., & Klockars, T. (2020). A Comparison of Four Digital Otoscopes in a Teleconsultation Setting. The Laryngoscope, 130(6), 1572–1576. https://doi.org/10.1002/lary.28340
United for Medical Research. (2025). NIH’s role in Sustaining the U.S. Economy.
Upadhyay, U. D., Zlidar, V. M., & Foster, D. G. (2016). Interest in self-administration of subcutaneous depot medroxyprogesterone acetate in the United States. Contraception, 94(4), 303–313. https://doi.org/10.1016/j.contraception.2016.06.006
US Centers for Disease Control and Prevention (CDC). (2021, May 21). Update to U.S. Selected Practice Recommendations for Contraceptive Use: Self-Administration of Subcutaneous Depot Medroxyprogesterone Acetate. https://www.cdc.gov/mmwr/volumes/70/wr/mm7020a2.htm
US Food and Drug Administration. (2020, October 22). FDA Approves First Treatment for COVID-19. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
Venkatraman, N., Silman, D., Bellamy, D., Stockdale, L., Bowyer, G., Edwards, N. J., Griffiths, O., Lopez, F. R., Powlson, J., Mair, C., Folegatti, P. M., Datoo, M. S., Morter, R., Minassian, A. M., Poulton, I., Collins, K. A., Brod, F., Angell-Manning, P., Berrie, E., … Hill, A. V. S. (2025). R21 in Matrix-M adjuvant in UK malaria-naive adult men and non-pregnant women aged 18-45 years: An open-label, partially blinded, phase 1-2a controlled human malaria infection study. The Lancet. Microbe, 6(3), 100867. https://doi.org/10.1016/S2666-5247(24)00083-1
Vogel, J. P., Wilson, A. N., Scott, N., Widmer, M., Althabe, F., & Oladapo, O. T. (2020). Cost-effectiveness of uterine tamponade devices for the treatment of postpartum hemorrhage: A systematic review. International Journal of Gynecology & Obstetrics, 151(3), 333–340. https://doi.org/10.1002/ijgo.13393
Wolf, J., Bruno, S., Eichberg, M., Jannat, R., Rudo, S., VanRheenen, S., & Coller, B.-A. (2020). Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens. NPJ Vaccines, 5, 51. https://doi.org/10.1038/s41541-020-0204-7
Wolf, J., Jannat, R., Dubey, S., Troth, S., Onorato, M. T., Coller, B.-A., Hanson, M. E., & Simon, J. K. (2021). Development of Pandemic Vaccines: ERVEBO Case Study. Vaccines, 9(3), 190. https://doi.org/10.3390/vaccines9030190
World Health Organization (WHO). (2020, April 1). Rapid communication on the role of the GeneXpert® platform for rapid molecular testing for SARS-CoV-2 in the WHO European Region. https://www.who.int/europe/publications/i/item/WHO-EURO-2020-1340-41090-55816
Yamashita, K., Taniguchi, T., Niizeki, N., Nagao, Y., Suzuki, A., Toguchi, A., Takebayashi, S., Ishikawa, J., Nagura, O., Furuhashi, K., Iwaizumi, M., & Maekawa, M. (2023). Cycle Threshold (Ct) Values of SARS-CoV-2 Detected with the GeneXpert® System and a Mutation Associated with Different Target Gene Failure. Current Issues in Molecular Biology, 45(5), 4124–4134. https://doi.org/10.3390/cimb45050262
Zhou, J., Blaylock, R., & Harris, M. (2020). Systematic review of early abortion services in low- and middle-income country primary care: Potential for reverse innovation and application in the UK context. Globalization and Health, 16(1), 91. https://doi.org/10.1186/s12992-020-00613-z
Zorzi, A., Cordioli, M., Gios, L., Del Bravo, P., Toskin, I., Peeling, R. W., Blondeel, K., Cornaglia, G., Kiarie, J., Ballard, R., & Mirandola, M. (2017). Field evaluation of two point-of-care tests for syphilis among men who have sex with men, Verona, Italy. Sexually Transmitted Infections, 93(S4), S51–S58. https://doi.org/10.1136/sextrans-2016-053065
Appendix: Methodology
Calculating funding flows
To calculate the flow of funding between different HICs, we used the G-FINDER survey data set for 2007-2023. For funding provided directly to product developers (as opposed to funding provided to intermediaries like PDPs, CEPI or the EDCTP) we used the G-FINDER recipient country. For direct funding for which no recipient nation was specified (around 10% of the total) we assumed that it followed the pattern of destination-specific funding, with 98% flowing to HIC recipients, with a geographical distribution mirroring the distribution of destination-specific funding.
For funding provided to (almost exclusively HIC-based) intermediary organisations like the EDCTP, we pro-rated the share of funding ultimately flowing to HICs based on the geographical distribution of funding provided by intermediaries, excluding destination unspecified funding from our analysis. This calculation resulted in our assigning 78% of funding to intermediaries to HICs, with that funding allocated as being received by the intermediary’s home country.
This outcome of this process suggested that 95% of HIC public global health R&D funding ultimately ends up in HICs. We viewed this figure as inflated because of its complete reliance on product developers’ home countries, ignoring the possibility of formal subcontracts or informal resource expenditures in LMICs by HIC-based institutions. Since none of this secondary funding flow is captured in the G-FINDER survey – though an indicative review of NIH subawards to LMICs identified only a relatively low level of formal NIH funding to LMIC-based recipients – we opted to lower our estimate of HIC recipient share from 95% to a relatively arbitrary figure of 90% to correct for this overestimation and to reflect our uncertainty about the exact figure. G-FINDER based funding flows from and two individual HICs were reduced by 5.3% to reflect this lower estimate of HIC recipient share.
Applying multipliers
Drawing on a thorough review of the academic and grey literature, we identified the economic multipliers applicable to each of the countries covered by our G-FINDER data to estimate the economic and scientific returns of two decades of global health R&D investment from HICs. For each study included in the review, we listed the GDP, job creation, and private sector multipliers, where available, and assessed whether the study fell within the scope of our analysis. We conducted this structured review across published studies and institutional reports, focusing on methodological robustness, relevance to health R&D, funding type (government or philanthropy), and time horizon (short- vs long-term). Multipliers were deemed in scope if they reflected credible, health-relevant impacts of public investment; others were excluded or qualified based on limitations such as reliance on input-output/IMPLAN models or other methodological shortcomings. We added annotations to each entry describing assumptions, strengths, and methodological considerations, ensured consistency in units and definitions.
Except for private sector investment – where we identified robust country-specific multipliers – we applied a common multiplier (with a defined lower and upper bound) across countries. Figures reported in the main analysis reflect the midpoint of this range. For the private sector, one study was used to estimate both the short- and long-term effects of public R&D investment on private sector response. While not specific to health R&D, this study is the most robust and detailed available, distinguishing between short- and long-term effects across specific countries and the OECD. Its findings were nevertheless corroborated by other country- or region-specific studies to ensure their validity.
We followed a similar methodology to estimate how many patents will be filed in response to two decades of HIC investment in global health R&D. Very few studies have assessed the cost per patent, likely due to the lengthy patenting process and the issue of endogeneity: organisations that are more successful at innovation tend to receive more funding and also file more patents, making it difficult to isolate the causal impact of funding on innovation. While patents are not a perfect measure of innovation, they remain the best proxy currently available. We identified two studies that examined the impact of research funding on patent output, both of which used instrumental variable approaches to address endogeneity concerns. In addition, the evaluation of the Horizon 2020 programme provides some indication of patenting activity resulting from public investment. However, it is important to note that the goal of that evaluation was not to estimate the cost per patent, partly because insufficient time had passed since the programme’s end, with many projects still ongoing at the time of review. That said, we divided the total investment by the number of patents filed as of the evaluation to derive an indicative estimate of the cost per patent, which turned out to be nearly ten times higher than the estimates from the two other studies. The patent figures presented in the report use the midpoint of the two in-scope studies, while the exact values from each study are used to inform the range.
Literature review to identify innovations
Economic multipliers don’t capture all benefits of investment in global health R&D. We conducted a literature review of innovations intentionally developed/tested/scaled for LMICs that ended up benefiting HICs to demonstrate the broad benefits of investing in global health R&D.
To identify the innovations with dual benefits, we leveraged in-house expertise and conducted systematic searches of academic and grey literature. Search terms included: ("low- and middle-income countries" OR LMICs OR "developing countries") AND ("biomedical innovation" OR "health technology" OR "point-of-care" OR "device" OR "diagnostic" OR "medical" OR "vaccine" OR "drug" OR "vector control") AND ("reverse innovation" OR “reciprocal innovation” OR "technology transfer" OR "health security" OR "economic spillover" OR "scientific contribution" OR "economic benefit").
Innovations were included if there was clear evidence that the innovation was originally intended for neglected diseases, emerging infectious diseases or sexual & reproductive health issues as defined by the G-FINDER survey scope. This included being first approved for, or initially investigated for, one of these global health areas, or significant advancements in the innovation were conducted for one of these global health areas or targeting LMICs.
We identified 22 innovations that met the criteria, spanning neglected diseases, emerging infectious diseases or sexual & reproductive health issues and product types (drugs, vaccines, diagnostics and devices). Select innovations were grouped based on commonalities and presented as case studies in the report.
• Vaccine adjuvants – AS01 and Matrix-M
• Women’s health – Mirena
• Innovations adapted for COVID-19 – ChAdOx1, SORMAS, remdesivir, GeneXpert, ERVEBO
• Repurposing innovations – eflornithine, BCG, hydroxychloroquine
Table of contents
- Key messages
- Interactive map of investment and benefits per HIC
- How do HICs benefit from investing in global health R&D?
- Understanding HIC investment in global health R&D
- What domestic returns do HICs see from investing in global health R&D?
- Leveraging global health R&D for all: The spillover effects of innovation
- Implications for the global health R&D ecosystem of the future
- Call to action
- References
- Appendix: Methodology