Irresistible: developing new solutions for antimicrobial resistant STIs
By Impact Global Health 8 September 2025
What this report tells us
- Antimicrobial resistance is a major public health issue, and a growing threat for a number of STIs, including gonorrhoea, trichomoniasis and Mycoplasma genitalium creating a major need for new treatments that can deal with resistant strains.
- Without improved diagnostics and vaccines though, even new antimicrobials will continue to quickly generate resistant strains, leaving us continually back at square one.
- Antimicrobial stewardship requires us to take a long term view; investing now in the products we need for the future and making better use of those we have today.
- The creation of ‘insurance policy’ second line treatments need to be properly incentivised and rewarded, even those which may never be widely distributed.
- Observational data allows product developers to bring repurposed products to market much more quickly, presenting another option to stock the arsenal for our AMR response.
Globally, rising levels of antimicrobial resistance (AMR) are threatening our ability to cure infections that have been treatable since the discovery of penicillin in 1928. The use, misuse, and overuse of a relatively small number of antibiotics have allowed bacteria to breed new strains that are resistant to existing treatments.
The rise of resistant bacteria (and fungi) mean doctors need to use existing antibiotics (and antifungals) carefully – ‘antimicrobial stewardship’ – to avoid accelerating the growth of resistance. This means only using antibiotics when necessary and choosing the right antibiotic for the right patient at the right time. But a lack of different treatment options and tools for detecting AMR mean doctors don’t have the means to do this effectively.
Ideally, clinicians should be able to tell, quickly and cheaply, exactly what strain their patient is infected with and what drugs they will respond to. Without this information, treatment requires guesswork, risking either the use of ineffective antibiotics on resistant strains, or wasting broadly effective second line treatments on ordinary cases. However, all too often, the diagnostics needed don’t even exist.
AMR also means that antibiotics we’ve relied on for decades no longer work. While effective diagnostics could help us use existing antibiotics more effectively to extend their working lives, we also need new antimicrobials that infectious diseases aren’t yet resistant to.
The three STIs covered in this report – gonorrhoea, Mycoplasma genitalium and trichomoniasis – are all displaying signs of resistance, with gonorrhoea already classified as ‘multidrug resistant’.
Untreated, or if they become untreatable, these STIs can spread easily through sexual contact and cause a variety of long-term effects, including complications in pregnancy or even complete loss of fertility. This report looks at the fight to contain rising resistance in these STIs: the remedies currently available, the funding for product development and the pipeline of products it has generated, and suggestions for how to manage R&D to minimise their risk of becoming untreatable.
Introduction
Researchers estimate that 1.27 million deaths directly resulted from bacterial resistance, globally, in 2019. The scale of this toll underlines the seriousness of AMR.
The WHO has identified three sexually transmitted infections (STIs) as being at risk of AMR: gonorrhoea, Mycoplasma genitalium and trichomoniasis, with the former classified as ‘high’ risk in its list of AMR bacterial priority pathogens, and all three imposing a disproportionate share of burden on women and girls. In response, the WHO has set an ambitious target of a 90% reduction in new gonorrhoea cases by 2030, while also raising the alarm about the other two – currently lower AMR risk – STIs.
In this report, we consider the future global response to AMR in STIs, by studying the funding that has been provided to research and develop new treatments, vaccines and diagnostics and the resulting product pipeline for each of the three infections and their disease-causing pathogens. By determining what products are on the horizon, and how far away they remain from the patient, we can arrive at a clearer picture of what the impact of STI AMR will be in the future, and what steps we need to take, today, to bring it under control.
Note: the pipeline data in this report is current as of Q4 2024 and incorporates major events through to Q2 2025, but does not reflect any more recent developments.
Funding for AMR
Since 2018, G-FINDER has collected R&D funding data on sexually transmitted infections with disproportionate burdens in low- and middle-income countries (LMICs), including the three AMR-risk pathogens that are the focus of this report. Data on gonorrhoea R&D funding is collected under a specific heading, while funding for the other two AMR-risk pathogens, Mycoplasma genitalium (‘Mgen’) and trichomoniasis, is captured under our umbrella ‘multiple and/or other STI’ category.
In total, just over $400m has been invested in R&D funding targeting STIs with AMR risk in the last six years, with total funding ranging from $60 to $70m per year. However, essentially all ($389m, 97%) research funding for STIs with AMR risk either focuses on or includes gonorrhoea. Funding exclusively focused on trichomoniasis totalled just $9m (2.3%), while Mgen-only funding was just $4.4m (1.1%).
The heavy skew towards gonorrhoea funding likely reflects a combination of factors. First is the greater AMR risk associated with gonorrhoea, notwithstanding the higher overall incidence and DALY burden from trichomoniasis. Second is the way we gather data on the three pathogens, with a separate category for gonorrhoea while trichomoniasis and Mgen are included under the catchall category ‘Other STIs’, potentially discouraging reporting of the funding they receive.
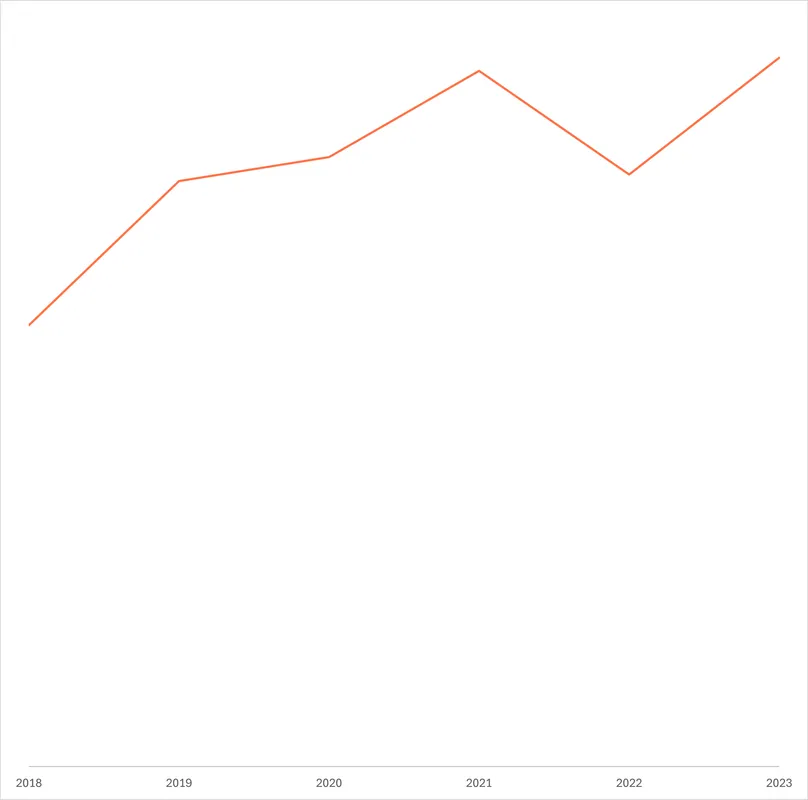
Funding for gonorrhoea jumped to a peak of $74m in 2023 (up $15m, 25%), after averaging $63m in the four years prior. Over the years, there has been a consistent shift towards gonorrhoea vaccine R&D, which rose from just $8.0m in 2018 to a record high of $42m in 2023 (57% of total funding). This shift towards vaccines is in part driven by the US NIH and the AMR-focused nonprofit CARB-X who have increasingly reallocated their funding from drugs to vaccines, in an attempt to prevent rather than only focusing on treating resistant strains. Rising vaccine funding also reflects a big rise in funding from industry – the other major funder of gonorrhoea R&D. Vaccine R&D is the only product area industry contributes to, perhaps reflecting a belief that this will become an increasingly profitable area in future unlike the development of new antibiotics. This increase in vaccine funding has helped support an increasingly advanced gonorrhoea vaccine pipeline, as we outline below.
Funding for new antimicrobials tends to be an area of particularly severe market failure, since private developers would likely anticipate relatively low returns on new second line therapies that are likely to be used only occasionally – an incentive problem we consider in more detail below. Since peaking in 2019, gonorrhoea drug R&D funding has declined for four consecutive years, taking 2023’s drug investment to a near record low. Shifts in the distribution of gonorrhoea funding have tended to reflect the timing and focus of CARB-X’s funding rounds, which focus on individual areas and cause a short-term spike in funding for successful applicants. So, in 2019, CARB-X’s $14m in funding for early-stage research including novel and first-in-class antibiotics followed its 2018 funding round for ‘new classes of direct-acting small molecule and large molecule antibacterials that target certain Gram-negative bacteria’. With later rounds focusing on other products and pathogens, CARB-X’s gonorrhoea drug funding has since fallen sharply – it has provided no drug funding at all since 2021. In 2023, following a 2022 call targeting a range of gonorrhoea products, CARB-X’s awarded funding focused on vaccines, in the form of $6.8m for the early-stage research of outer membrane vesicle and multivalent vaccines.
One major success story in the search for novel gonorrhoea antimicrobials is the first-in-class oral antibiotic zoliflodacin. Zoliflodacin was the primary beneficiary of the $22m the Global Antibiotic Research & Development Partnership (GARDP) has invested in gonorrhoea drug R&D since 2019. It released successful Phase III trial results in 2023 and in June 2025 was granted Qualified Infectious Disease Product status by the US FDA, entitling it to priority review for use against gonorrhoea. This represents proof-of-concept, not just for zoliflodacin, but also of GARDP’s model of antimicrobial development, since it looks set to become their first approved product from their substantial portfolio of antimicrobial candidates.
While drug funding has slumped, gonorrhoea basic research funding reached a record high after new contributions from the philanthropic sector, which historically has been very limited. In 2023, the Gates Foundation reported a $7m grant for basic research focused on proof-of-principle for a vaccine.
Funding for biologics and diagnostics R&D experienced slight increases in 2023, rebounding from record lows in 2022, although funding for both areas remains much lower than in 2019. Without backing from industry, both diagnostics and biologics product development looks anaemic compared to drugs and vaccines, despite the critical importance of pairing new therapeutics with diagnostics to identify resistant strains and support antibiotic stewardship.
Figure 1 – Gonorrhoea funding by product type
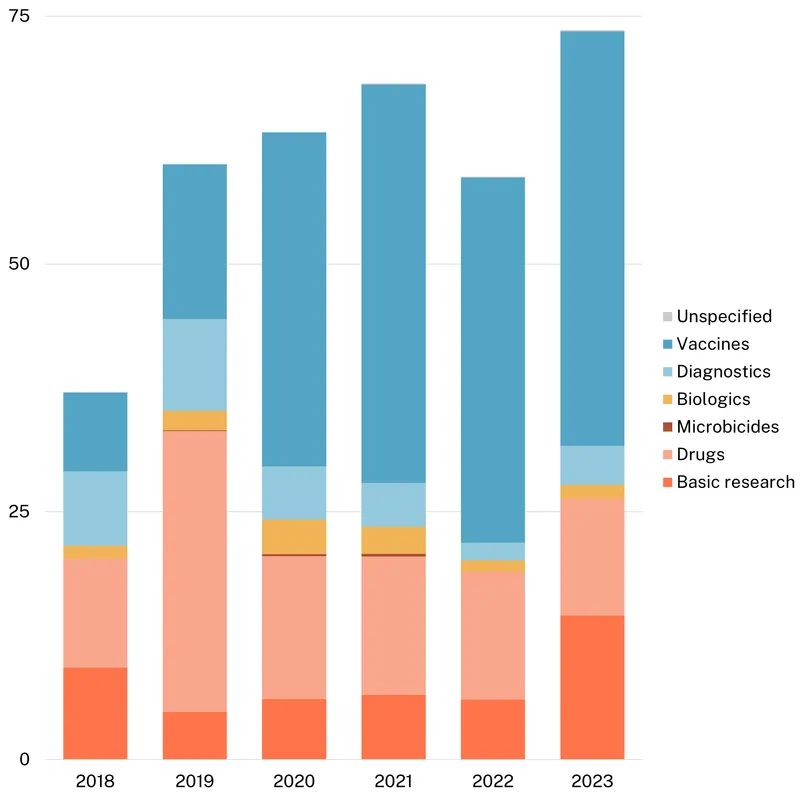
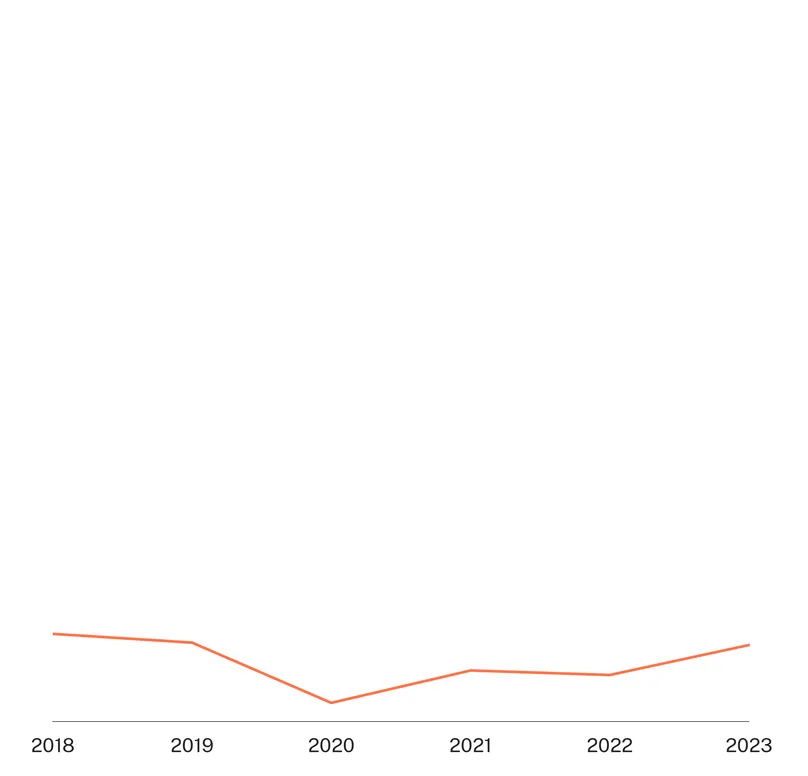
There was only a small amount of funding specifically targeting trichomoniasis, which currently displays lower levels of AMR than gonorrhoea, despite its much higher global prevalence. Trichomoniasis funding rebounded slightly in 2023 (up $0.5m, 65%) linked to increased basic research and diagnostics funding. This increased funding came entirely from the US NIH, which was responsible for nearly all trichomoniasis funding in 2023. As a result, fluctuations in funding levels and product focus for trichomoniasis are all largely driven by the direction of US NIH funding, leaving it highly vulnerable to reductions in NIH funding, especially those that target projects focused on women’s health. This is particularly true of the long term decline in the share of drug R&D, which fell from 72% of the total to 49% between 2018 and 2023.
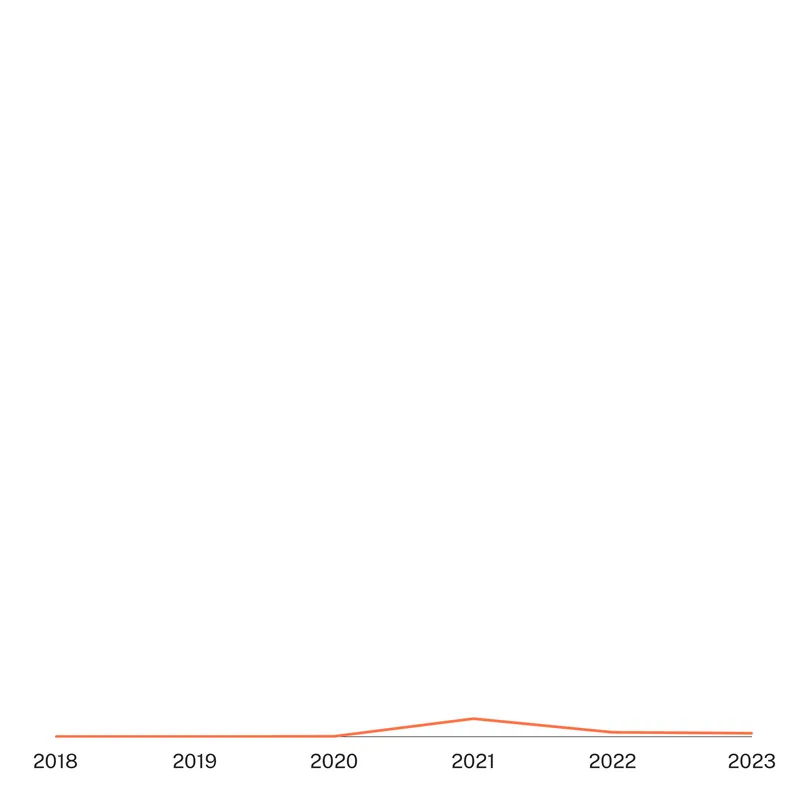
There has been very little funding specifically targeting Mycoplasma genitalium. What little funding that was reported has now fallen for a second straight year after its (unimpressive) 2021 peak, but remained above the lows it experienced from 2018 to 2020, when little or no funding was reported. The peak in 2021 was linked to first time industry reporting for molecular platform diagnostic development, funding which fell to zero in 2023, leaving the US NIH as the sole remaining funder of Mgen R&D.
Funding targeting more than one STI with AMR risk represented less than $100k in 2023. Research in this area has largely focused on the development of a molecular high throughput test to diagnose chlamydia, gonorrhoea and trichomoniasis simultaneously, which preliminary data shows will rise substantially in 2024 thanks to a new round of funding from CARB-X.
Industry’s relatively healthy funding for gonorrhoea vaccine R&D, and its lack of interest in either of the other two AMR-risk STIs, is noteworthy. The gap seems to reflect differences in the real and perceived threat from AMR, with trichomoniasis having almost twice as many new cases (156.3 million in 2020) but gonorrhoea incidence currently growing more rapidly and displaying more widespread resistance, leading to its categorisation as a ‘high’ threat bacterial pathogen – the second highest threat level – in the latest WHO AMR bacterial pathogen priority list. This lack of overall investment – in drugs, vaccines or from industry – is a problem for trichomoniasis, for which there is currently only one class of effective antibiotics, meaning there is no available fall-back strategy for strains resistant to that class, nor much in the pipeline to fill that gap in the near future.
What products are being developed?
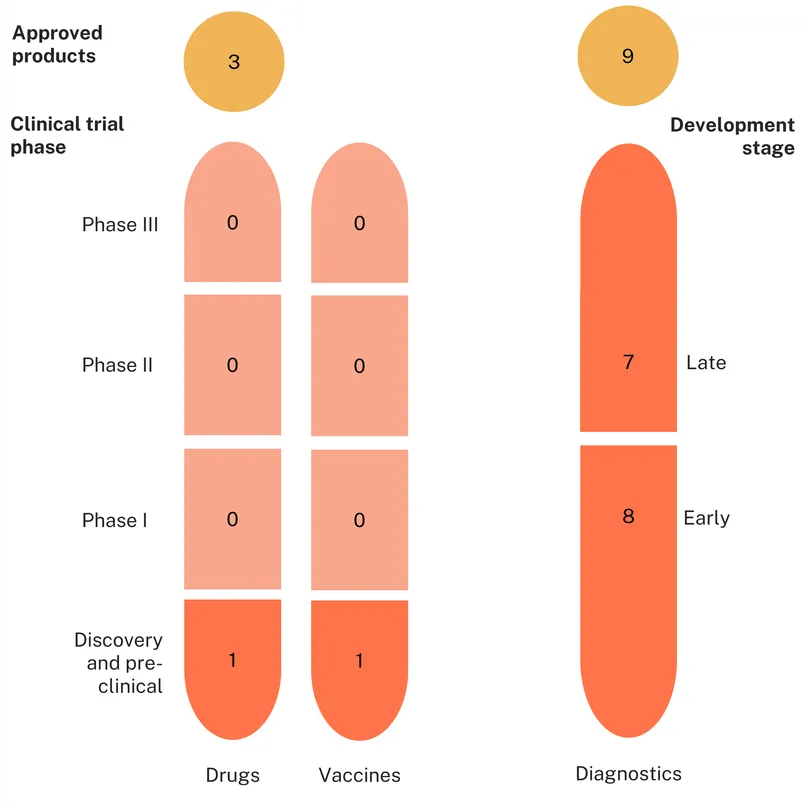
Our review of the pipeline (based on the methodology outlined here) revealed a total of 164 candidates in the pipeline and 52 approved products for STIs with AMR risk. Of these, 129 were for gonorrhoea (109 candidates and 20 approved products), 58 for trichomoniasis (38 candidates and 20 products), and 29 for Mycoplasma genitalium (17 candidates and 12 products).
There are currently no approved vaccines, biologics or microbicides for any of these STI, only diagnostics (36) and drugs (16).
Gonorrhoea
The existing gonorrhoea product landscape comprises 20 approved products, of which six are drugs and the rest diagnostics. Currently, there are no gonorrhoea vaccines approved by the WHO or national regulatory bodies, though in May 2025 the UK government announced the pre-approval rollout of the 4CMenB repurposed meningococcal vaccine – discussed further below – in response to record case numbers in the UK.
The absence of a widely approved vaccine, and the limited number of viable therapeutics, has meant that drug-resistant gonorrhoea is a growing problem, with antimicrobial resistance currently reported against almost all the currently recommended treatments. For some drugs, such as ciprofloxacin, the vast majority of submitted samples (96%) from countries that reported data to the Enhanced Gonococcal AMR Surveillance Programme (EGASP) were found to be resistant. Even for drugs such as ceftriaxone, which is the mainstay of gonorrhoea treatment, at least two countries, Cambodia and Vietnam, have reported rates of resistance over 15%, far above the 5% threshold at which WHO recommends discontinuing the use of an antibiotic as a first-line treatment.
Cheap, quick and easily accessible diagnostics play an essential role in avoiding the unnecessary use of antibiotics. However, only three of the approved gonorrhoea diagnostics are able to be used at or near the point of care (POC); the remainder all require sophisticated laboratory infrastructure, and there are no commercially available rapid diagnostic tests for detecting gonorrhoea. More importantly, only one test can assess antibiotic susceptibility, and even that only covers one class of antibiotics.
Figure 2 – Proportion of isolates with resistance to ciprofloxacin reported to WHO-GASP
There are 109 different biomedical countermeasures in development for gonorrhoea. Most of these technologies – more than three-quarters of the total – are either diagnostics (44) or drugs (41). Another fifteen are vaccines, along with eight biologics and just one microbicide.
The gonorrhoea diagnostics pipeline is mostly in its early stages: only nine (out of 44) tests have reached late-stage development, and only two of these have the potential to test antibiotic susceptibility. New diagnostics are urgently needed that can be used at or near the point of care to provide faster results that can guide doctors to administer the right antibiotics to the right patient. Without diagnostics capable of detecting resistant strains quickly enough to be able to inform immediate prescribing decisions, it will be impossible to preserve the remaining effectiveness of existing antibiotics (primarily ceftriaxone) or to make the most of potential new ones.
As with diagnostics, gonorrhoea’s therapeutics pipeline is comprised mainly of candidates in the early (discovery and preclinical) phases. Just three therapeutics candidates have reached clinical development, two of which are groundbreaking first-in-class antibiotics, with the remaining clinical candidate a repurposed drug, ertapenem. One of these, GSK’s gepotidacin, is currently under priority review by the US FDA having already received approval for urinary tract infections early in 2025. This reflects gepotidacin’s status as arguably the most promising of the three, with positive results from its Phase III trial early in 2024 demonstrating noninferiority to the existing regimen of ceftriaxone injections combined with oral azithromycin – offering functionally identical cure rates and a slightly worse side effect profile. The other first-in-class candidate, zoliflodacin, jointly developed by GARDP and Innoviva, has also successfully completed a pivotal Phase III trial, which showed non-inferiority when compared to the standard ceftriaxone/azithromycin regimen. Based on these results, zoliflodacin’s new drug application was accepted by the US FDA in June 2025, starting it on the path towards approval. In addition to providing new options for use against resistant strains, both of these potential new gonorrhoea treatments can be taken orally, an improvement on the current gold standard regimen that requires injections.
While it’s a relief to have new therapeutic options on the horizon, maximising their useful lifespan means ensuring new treatments are used appropriately. Ideally, this means moving away from the current practice of using a clinical algorithm based on local resistance patterns to determine antibiotic use, in favour of diagnostic-guided treatment. If we launch new antibiotics before we have the necessary diagnostics in place, we will repeat the process of selecting for resistance, putting ourselves back in the same situation in a decade, with – as things stand – little in the way of alternative candidates to fall back on considering the vast majority of the remaining pipeline, including potential vaccines, is at best at the concept stage.
One potential means of extending the lifespan of new and existing therapeutics is the development of gonorrhoea vaccines. An effective vaccine is key to achieving the WHO’s target of reducing gonorrhoea incidence by 90% by 2030, which would require a novel vaccine to provide at least 52% protection for at least six years. The search for a gonorrhoea vaccine has been frustrated by the ability of gonorrhoea-causing bacteria (Neisseria gonorrhoeae) to evade the body’s natural immune response and the resulting inability of natural infection, or a vaccine that’s simulates it, to provide protective immunity. Recently, several observational studies have shown that serogroup B meningococcal (Men B) vaccines may offer cross-protection against gonorrhoea; this has reignited optimism that one or more of the existing Men B vaccines – already with proven safety profiles and ability to be produced at scale – might form the basis of an effective gonorrhoea vaccine.
The fifteen vaccines in the gonococcal vaccine pipeline includes two candidates in clinical development. The most advanced is the 4CMenB repurposed Men B vaccine recently slated for distribution in the UK based on observational data. Notwithstanding its pending use in the UK, it remains in Phase III trials for use against gonorrhoea and, if the roughly 30% efficacy observed to date can be replicated in real world conditions, it has the potential to decrease gonorrhoea burden globally by 45% by 2030. The second vaccine in clinical development is GSK’s GAMMA platform-based candidate, which is undergoing a Phase II proof-of-concept study. The remainder of the gonorrhoea vaccine candidates are all still in early-stage development. Given their current status, though, none of these early-stage candidates represent a real possibility for hitting the WHO’s 2030 target, leaving us with only two shots on goal.
The remaining gonorrhoea pipeline consists mainly of immunotherapeutics, most of which are in the early concept stage, and only one candidate, GneX 12 – a sustained-release formulation of interleukin-12 – is close to filing with the US FDA for the ‘investigational new drug’ (IND) status that would enable human trials to begin. The single microbicide in the pipeline, Yaso-GEL – a multipurpose prevention technology product geared specifically towards women and girls as a vaginal gel offering the potential for simultaneous protection against gonorrhoea, HSV-2 (genital herpes) and pregnancy – has shown promise in mouse-model studies, but remains a long way from potentially reaching the market.
Figure 3 – pipeline candidates and approved products for gonorrhoea
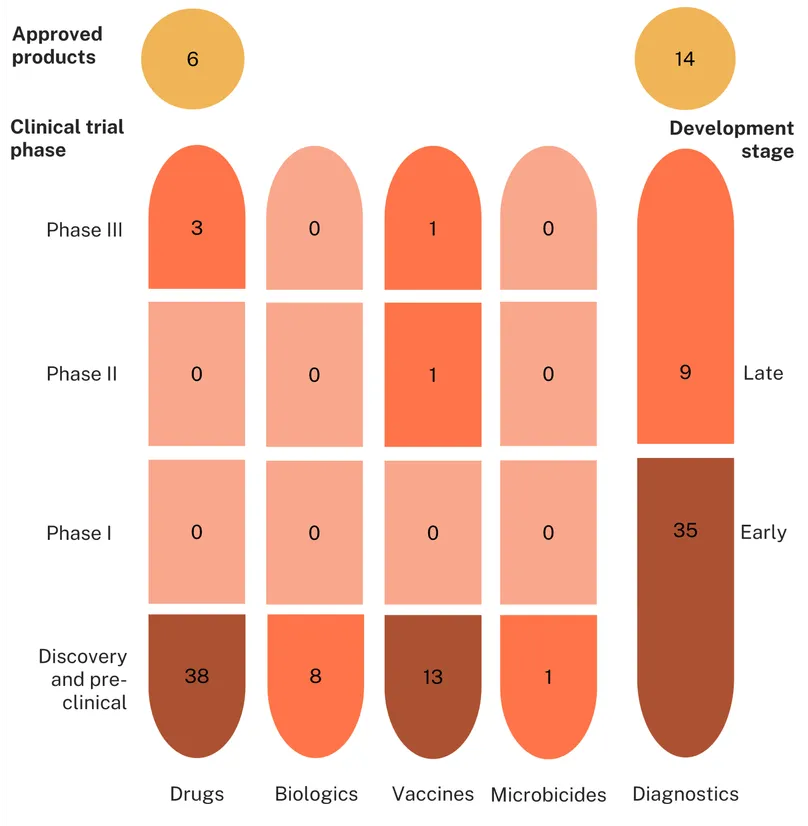
Trichomoniasis
The landscape of approved products for trichomoniasis includes thirteen diagnostic tests, seven drugs, and, as with gonorrhoea, no approved vaccines.
The thirteen diagnostics currently available to detect trichomoniasis-causing T. vaginalis include ten molecular (NAAT) tests, a pair of immunoassays, and one microscopy-based test. There are approved POC tests and, unlike gonorrhoea, approved rapid diagnostic tests. Like gonorrhoea, trichomoniasis also enjoys coverage from multi-STI diagnostics designed to detect more than one STI, including the recently approved, CARB-X backed, Visby Medical Women’s Sexual Health Test – the first diagnostic test approved by FDA for chlamydia, gonorrhoea and trichomoniasis and which can be purchased without a prescription and performed entirely at home. Despite the promising range of diagnostics options available, none of them offer fast or local antibiotic susceptibility testing, which still requires sending samples to reference laboratories.
Currently recommended treatments for trichomoniasis, such as metronidazole, all belong to the nitroimidazoles class of drugs. Although resistance is not yet widespread, there have been increasing reports of antimicrobial resistance and treatment failure associated with metronidazole. This makes it particularly concerning that the alternative second-line medicines all belong to the same class of drugs, making them more vulnerable to cross- resistance developed from exposure to metronidazole. The other problem with metronidazole is the requirement for patients to take a seven-day course, which can cause adherence issues, speeding the development of resistance.
The trichomoniasis product development pipeline includes 38 candidates – just over half of which (21) are drugs. Almost all the remaining candidates – another 16 – are diagnostics, with just one active vaccine candidate in the pipeline.
As suggested by our funding data, and unlike the relatively advanced drug pipeline for gonorrhoea, all the current drug development for trichomoniasis is still stuck at the discovery/concept stage. There is not a single candidate undergoing the kind of advanced preclinical studies necessary to begin clinical testing. This lack of progress has persisted in spite of the WHO’s global STI research priorities, which call for improved trichomoniasis therapeutic options, including drug-resistant infections. It is both concerning and unclear as to why trichomoniasis – a known AMR risk, the most common non-viral STI, and a risk factor for HIV transmission – receives almost no funding for drug development. One potential ray of hope is a $9.2m 2024 grant from the US NIH (not yet captured in our funding data) for a study of secnidazole, an already approved treatment for trichomoniasis, against the current standard of care. The study aims to test whether a one dose regimen improves compliance enough to make it a superior practical alternative. Continuation of this line of NIH funding in 2025 appears precarious however, given the recent wave of project terminations, especially those focused on the health of women and girls.
The trichomoniasis diagnostics pipeline mirrors drug development, with 13 (out of 16) tests still in early development. All but three of these diagnostics are molecular, including several based on non-traditional platforms such as CRISPR and LAMP. Only one diagnostic candidate is designed to test antimicrobial susceptibility, greatly limiting the potential usefulness (and lifespan) of any new treatments, if and when they are developed. Since drug resistance testing is not currently widely available, diagnostics development efforts need to focus on tests that can be performed at the point of care and which, along with diagnosing trichomoniasis infection, can test for the presence of resistant strains.
The trichomoniasis vaccine pipeline is almost non-existent, with only one candidate, based on a subunit approach, which is still at the concept stage and yet to undergo any in vivo studies.
Figure 4 – pipeline candidates and approved products for trichomoniasis
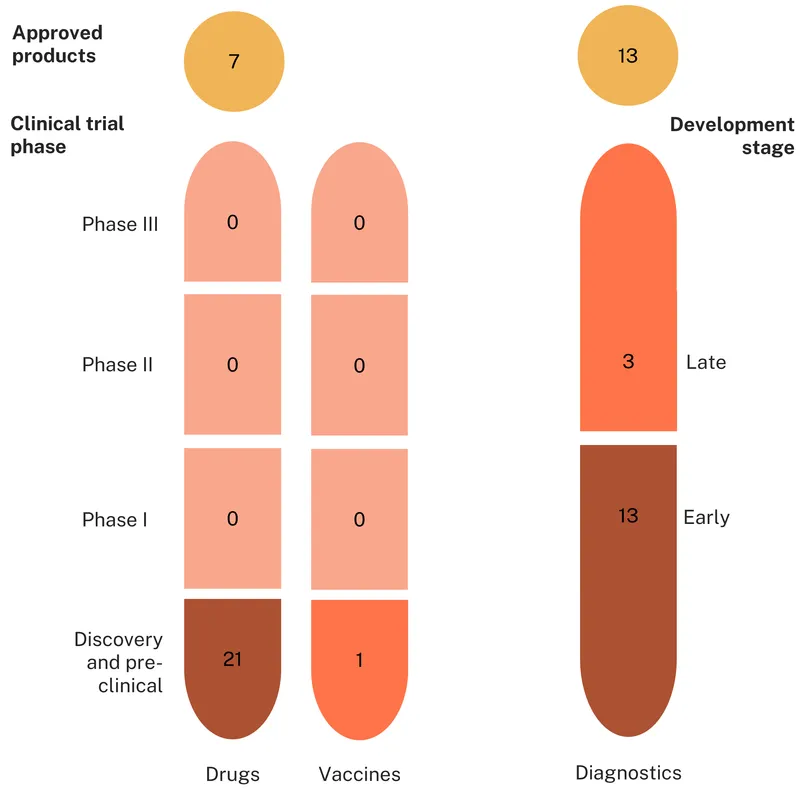
Mycoplasma genitalium
There are currently nine approved diagnostics, three approved drugs and zero approved vaccines targeting Mycoplasma genitalium.
Molecular testing is currently the gold standard for detecting Mgen infection. All nine approved diagnostics are based on a molecular platform, including two tests capable of detecting antimicrobial resistance and one POC test. No serological tests, such as antigen-based or lateral flow assays, have so far proven effective in diagnosing Mgen infections.
The two recommended therapeutic options currently available to treat Mgen – macrolides and fluoroquinolones – are already experiencing significant levels of antimicrobial resistance. As a result, the WHO recommends varying treatment based on the patient’s AMR profile, alternating between the two primary therapeutics – azithromycin and moxifloxacin – based on resistance. However, access to diagnostics capable of detecting resistance is often limited in LMICs and other low resource settings, requiring medical staff to fall back on local surveillance data as a proxy for the likely risk of resistance in their patients.
As suggested by the low levels of funding outlined above, the Mgen product pipeline is relatively small. There are just 17 products in development, the vast majority of which (15) are diagnostics. Drug and vaccine development is almost non-existent, with only one candidate in each category.
As with existing diagnostics, tests currently in development for detecting Mgen, including those for antibiotic susceptibility, are primarily based on a molecular platform. Close to half of the diagnostics pipeline has, at least, reached late stages of development, including four new tests with the capacity to detect antibiotic susceptibility – which is promising.
The lone drug (lefamulin, a semi-synthetic pleuromutilin antibiotic) and one vaccine (a multi-epitope subunit vaccine) currently in development both remain in their early stages. Considering the rising antibiotic resistance profile of Mgen, this lack of drug development effort is a major concern.
Figure 5 – pipeline candidates and approved products for Mycoplasma genitalium
What can we learn from the landscape?
Without improved diagnostics and vaccines, even excellent new antimicrobials will continue to quickly generate resistant strains, leaving us back at square one
Stewardship of new and existing antimicrobials requires us to provide them only when they are likely to offer a successful cure. This requires, first, that antimicrobials are used only when the patient actually has the disease in question, and, second, that the right antimicrobial is prescribed for the infection.
The existing product landscape for all three STIs covered in this report displays significant gaps for both use cases. There are too few rapid, point of care, tests capable of immediately identifying the disease-causing pathogen for any of these three infections in low resource settings. And there are even fewer of the advanced diagnostics necessary to identify which treatment a patient ought to receive. These gaps drive overuse of first line therapies – to patients with resistance or patients with another disease entirely. It also drives overuse of second line therapies, because clinical guidelines based on population level antimicrobial resistance data recommend them for all patients, even those who don’t have resistant infections.
As things stand, the development of new therapeutics, while still behind where we need it to be, has outpaced the creation of the diagnostics needed to make best use of them. If novel drugs are launched into a world where clinicians have to guess when to use them, the cycle of resistance will start again. We need a concerted effort from funders to align the timeline for diagnostics development to the potential launch of new treatments, ideally by incorporating diagnostics development into the drug clinical development programme.
In concert with an increased focus on diagnostics, viable vaccines would help buy us time. Reduced rates of infection could extend the remaining lifespan of existing therapeutics, and allow us to properly steward the application of new ones, possibly even achieving a stable situation where strains resistant to one antimicrobial can be eliminated using a different one, and averting the development of multi-resistance. Despite the uptick in gonorrhoea vaccine funding from industry, and the UK’s pre-registration use of repurposed meningitis vaccines for gonorrhoea, overall, the lack of any widely-approved gonorrhoea vaccine – and with just two in late stage development – could end up being a missed opportunity.
Antimicrobial stewardship requires us to take a long view; investing now in the products we need in the future and making better use of those we have today
Developing new medicines, vaccines and (to a lesser extent) diagnostics is unavoidably slow, especially when the lack of a large-scale commercial market means that there isn’t enough money to go around. Below, we present some suggestions for providing proper incentives and making the process move a little faster, but even if they were adopted, it would still take many years to come up with a new antibiotic for gonorrhoea.
The lag between when we realise we need new drugs and when we can begin administering them means that we need a long-term plan for dealing with AMR. Organisations like CARB-X and GARDP provide a useful coordinating function for product developers – helping identify the most promising approaches and supporting them across the several ‘valleys of death’ that plague product innovation. With a clearer understanding of how long it will take to gain access to new therapies, we can better allocate funding across the pipeline and better safeguard the antimicrobials we have today.
This last obligation, antimicrobial stewardship, is somewhat beyond the scope of this report, but still an incredibly important part of how we manage the wait for new treatments. With a clearer understanding of R&D timelines and the dynamics of resistance it should be possible to more accurately optimise dosages and the threshold of resistance at which we reach for second line therapies. The existing 5% benchmark has sat unchanged for more than 20 years, relies on narrow calculations about differences in antibiotics’ cost to the health system, and is mostly ignored in high-income countries in favour of a – similarly arbitrary – 3% standard. There are of course optimal levels of resistance at which we should begin deploying second line therapies, this number is vitally important to preserving our existing treatments, but it is almost certainly neither exactly 5% nor exactly 3% and definitely not both. While we wait for advanced POC diagnostics which will allow clinicians to offer individually tailored treatment, we should review the guidelines for optimal treatments and dosages when we lack access to perfect information, tailoring them to the expected wait for new ones.
The creation of ‘insurance policy’ second line treatments needs to be properly incentivised and rewarded, even for those which may never be widely distributed
Compensating product developers on a fee-per-dose or fee-for-service basis for second line treatments will undoubtedly lead to underinvestment, especially given if they work as intended, they may never require mass distribution. In many cases, second line treatments are not approved, or do not even exist yet, because they would be inferior to existing treatments in a first line role. A drug like solithromycin for uncomplicated gonorrhoea, which demonstrates marginally less efficacy (80% vs 84%) at trial and a slightly worse side effect profile is a largely worthless commercial prospect today – and for at least the next five years – while representing an incredibly valuable insurance policy for sufferers infected with resistant strains and for the preservation of the existing – and still profitable – first line regimen.
Regulators could relax their standards for approving treatments which are inferior to existing standards of care – from the existing benchmarks of noninferiority at up to 10 or 12% lower efficacy – to allow space for therapeutics we might need increasingly often in the future, while health systems need to incentivise and reward developers who foster a diverse product landscape, as a collective insurance policy against future AMR.
In addition to greater regulatory flexibility, health systems could build innovative funding models suitable for rarely used, but highly valuable antimicrobials, so that developers can recoup costs even if their product remains a second-line treatment. This could resemble the UK’s ‘Netflix’ subscription model for antimicrobials, whereby the UK’s National Health Service pays developers a fixed annual fee for access to its antimicrobials, regardless of how many doses are used, or the minimum revenue guarantees being considered by the EU.
Observational data allows product developers to bring repurposed products to market much more quickly, presenting another option to stock the arsenal for our AMR response
The UK’s recent approval of the repurposed meningococcal vaccine, 4CMenB, for use against gonorrhoea in response to spiking cases numbers and some early evidence of resistant strains, shows a promising avenue to tackle AMR-risk STIs. Even at an estimated 33-47% efficacy – below the WHO’s suggested benchmark of 50% – providing high risk populations with 4CMenB could avert as many as 100k cases over the next decade, reducing the use of existing first line therapies and buying time for the development of new ones.
The other key takeaway from this story though, is how 4CMenB went from Phase III trials in April 2025 to a national rollout in May of the same year. Trials, especially vaccine trials, tend to move slowly. Once they are completed, regulators typically spend significant time reviewing results. The 650 person (very small, in vaccine terms) Phase III trial of 4CMenB being conducted in Australia began in 2021, and isn’t scheduled to conclude until October of 2025. To successfully demonstrate a conventional statistically significant reduction in incidence, given a vaccine which is actually around 36% efficacious, about a quarter of the control would have to catch gonorrhoea over the life of the study. Which is more than a thousand times the rate of incidence across Australia. Even targeting a high-risk population – as this study’s designers have – proving a vaccine works is slow, demanding, expensive work.
How the UK government satisfied itself that 4CMenB helps to prevent gonorrhoea was quite different. They drew on observational data, primarily from New Zealand, which showed that when people received a vaccination for meningitis, they were significantly less likely to subsequently develop gonorrhoea. On this basis, the UK government decided that giving high risk populations access to a repurposed vaccine known to be safe and strongly suspected to be effective today was, on balance, more cost effective than waiting an extra year or more to be absolutely sure.
We argue above that AMR is moving at a speed that our product development efforts are struggling to match. There is little value in setting thresholds of resistance at which clinicians should switch to second line antimicrobials if there are no second line products to switch to. And there is little point in developing those new therapeutics if diagnostics can’t tell us which patients to give them to. Late-stage clinical trials are an incredibly valuable source of evidence to show which products actually work, but they are not the only source of evidence especially when we are losing the race against resistance. In order to address the growing burden from resistant STI strains – a burden that falls especially heavily on women and girls – we can take lessons from the UK, and prioritise speed over certainty.
Download the PDF of the reportAnnex: briefing on Irresistible
Briefing on Irresistible
This briefing summarises the key messages from our Irresistible report on the future global response to three AMR-risk STIs: gonorrhoea, trichomoniasis and Mycoplasma genitalium. It highlights current R&D funding for treatments, vaccines and diagnostics, and assesses the product pipelines for each pathogen.