Maternal Health
Postpartum haemorrhage (PPH)
Overview
Postpartum haemorrhage (PPH) is the leading cause of maternal death globally, affecting approximately 14 million women annually and resulting in around 70,000 deaths each year. PPH is defined by blood loss exceeding 500 ml after birth. Over 70% of cases are caused by a lack of muscle tone or contractions (‘atony’) in the uterus following delivery, with smaller shares due to trauma (such as genital tract lacerations), part or all of the placenta remaining in the uterus after childbirth, and impaired blood clotting. Severe bleeding may require emergency surgical intervention, and survivors can face lifelong repercussions, including anaemia, cardiovascular issues, reproductive disabilities, as well as mental health challenges. Management of PPH requires early identification and rapid intervention to stabilise the patient's blood flow and pressure, usually through administration of uterotonics, with oxytocin the gold standard to treat PPH due to uterine atony, as well as management of blood loss through intravenous replenishment of body fluids. If bleeding persists or if oxytocin or other uterotonics are not available, additional interventions may include bimanual massage to stimulate uterine contraction, as well as devices such as uterine tamponades to apply internal pressure, or sutures to control bleeding from specific sites.
Unmet needs
In high-income countries, most births occur in places where uterotonics and surgical interventions are widely accessible, contributing to generally low rates of maternal death from PPH. However, while medicines are the cornerstone of PPH prevention and treatment, the current catalogue is suboptimal for LMIC settings, where difficulties with product quality, cold-chain transport and storage, and skilled administration limit access. Research focused on heat-stable and inhalable or sublingual uterotonics is critical in addressing these disparities, but there are currently only three heat-stable medicines available. Prophylactic use of medicines (drugs, like carbetocin and tranexamic acid, and dietary supplements) could also contribute to limiting incidence and the need for emergency interventions, provided they can be administered in all settings, but more research is needed to establish efficacy. Low-tech bleeding-control devices can also serve as an important intervention when medications fail or are unavailable, with some (uterine balloon tamponades) currently recommended by the World Health Organisation for treatment of PPH in women who do not respond to standard first-line treatment. Many of these devices still lack strong evidence of efficacy, and require training and availability to be effective.
Funding
Global funding for PPH rebounded to a record $11m in 2022 before declining by 21% to $8.6m in 2023.
Falls in PPH funding after 2018 were the result of declining industry funding, which fell from over $7m in 2018 to just $0.8m in 2021 before rebounding a little in 2022.
With industry funding declining again in 2023 following the successful completion of African heat-stable carbetocin trials, the rebound in overall PPH funding was instead driven by a new line of drug funding from Unitaid beginning in 2022. Unitaid provided more than $6m in both 2022 and 2023, mostly for the ‘Accelerating Measurable Progress and Leveraging Investments for Postpartum Haemorrhage Impact’ (AMPLI-PPHI) implementation trial, which aims to demonstrate the relative feasibility and cost-effectiveness of heat-stable carbetocin, tranexamic acid, and misoprostol in various LMIC settings.
The other major factor in PPH funding’s rebound from its record low in 2021 was the 2022 commencement of funding from the US NIH. It contributed a total of $1.6m over the last two years, for a mix of drug and device R&D. The NIH’s drug R&D includes $571K in funding for OPTIMUM OB-TXA, to investigate the optimal timing, route, and dose of tranexamic acid (TXA) before umbilical cord clamping; while it’s device funding backed the first definitive randomised controlled multicentre trial of the FDA-approved ‘Jada system’ in Ghana, gathering critical data on its effectiveness, safety and cost effectiveness at treating PPH.
Drug R&D continues to account for the vast majority of PPH funding: 89% of the 2023 total and over the life of the survey, with the remaining 11% going to devices and device/drug combinations. In contrast to most other areas of maternal health, funding for basic research into PPH is not included in our survey since the physiology of the condition is well understood.
Funding for PPH


Product landscape & pipeline
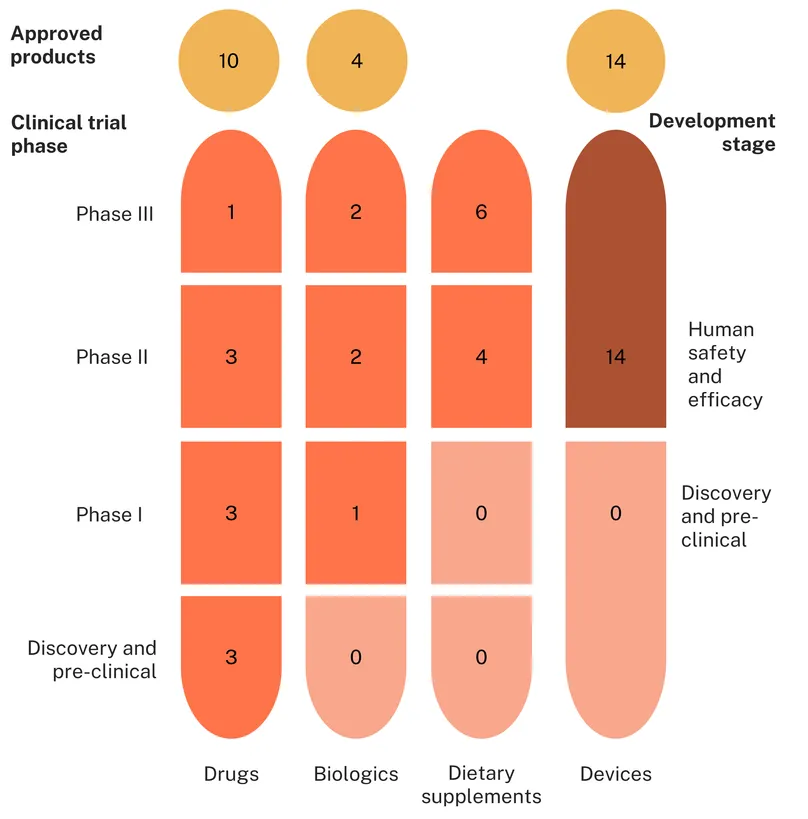
PPH is somewhat distinct from other pregnancy-related conditions – a mechanical blood loss rather than a progressive pathology, meaning the biomedical landscape looks quite different too. The R&D pipeline for PPH is generally smaller than for other maternal health conditions, with only 25 candidates in development compared to 67 for preeclampsia and 75 for preterm labour, but with a greater number and proportion of marketed products available (14 in total). Medicines such as uterotonics and haemostatics are the cornerstone of PPH management. However, accessibility of these medicines can be limited in low-resource settings. The pipeline, therefore, also contains a number of low-tech devices in use or development, more appropriate for LMIC settings.
Thirty-nine candidates and products are in use or in active development for postpartum haemorrhage, including 20 drugs, nine biologics and 10 dietary supplements.
A little over a third of these are products marketed or adopted (14, 36%), including six repurposed medicines (two drugs, four biologics) and eight drugs that are new chemical entities (NCEs). All approved biologics are haemostatics and coagulants developed to treat general haemorrhage and applied to obstetrics for PPH: recombinant von Willebrand factor, eptacog alfa (initially developed for patients with haemophilia), fresh frozen plasma (including cryoprecipitate) and platelets.
Most approved drugs are uterotonics, reflecting the predominant underlying cause of postpartum haemorrhage being uterine atony. These are specifically indicated for PPH and include several formulations of oxytocin (alone or combined with ergometrine) or its long-acting synthetic analogue, carbetocin, with a heat-stable formulation applicable to low-resource settings where cold chain storage can’t always be guaranteed. When treatment with oxytocin is ineffective, prostaglandins and prostaglandin analogues are available as a second-line treatment, such as carboprost, and sulprostone. In this category of drugs, dinoprost, a synthetic analogue of the naturally occurring prostaglandin F2 alpha, used to be an established second-line treatment but has been discontinued worldwide, with only limited supply available, and is therefore not included in this analysis.
Another prostaglandin analogue, misoprostol, is widely used as a uterotonic and recommended by the WHO, notably because its heat-stable, oral formulation makes it applicable to all settings. However, the association of the drug with abortion sometimes limits its availability and uptake in contexts that are hostile to the procedure, even for distinct obstetric and gynaecological purposes. The antifibrinolytic tranexamic acid, used to treat or prevent excessive blood loss from major trauma and surgery, is recommended by the WHO when uterotonics fail to control the bleeding, but requires skilled health workers for intravenous administration. Finally, ergometrine, an ergot alkaloid that promotes uterine and vascular smooth muscle contraction, is recommended as an effective uterotonic, but its side effects include an increased risk of hypertension. Overall, the catalogue of available medicines is not entirely fit for purpose, with only three heat-stable formulations and supply and safety limitations for several of them.
Six repurposed drugs are being investigated for PPH: the sclerosing agent polidocanol which recently started Phase I, the haemostatic agent etamsylate (Phase II), the vasoconstrictors vasopressin and terlipressin investigated in high-risk instances (Phase II) and the antibiotic metronidazole, investigated in combination with misoprostol and oxytocin in a Phase III study in Iran. The antihypertensive propranolol is investigated for its potential to resensitise the myometrium to oxytocin (preclinical). There are only four NCEs in development, signalling few true innovations, but all of them are suited for LMIC settings: a new inhalable therapeutic, CR003, in preclinical development by Crystec Pharma, and three different heat-stable formulations of oxytocin (inhaled, micro-array patches and orally/disintegrating).
Unsurprisingly, four out of five biologics in development are repurposed coagulation factors indicated for other bleeding disorders, such as Factor XIII concentrate (also known as Fibrin Stabilizing Factor) and Fibrinogen concentrate in Phase III, and Prothrombin complex concentrate recombinants and a fixed dose combination of von Willebrand factor/coagulation factor VIII in Phase II. Interestingly, CT 001, a next-generation recombinant and activated version of factor VIIa is being developed specifically for PPH as a new chemical entity. However, while expected to enter Phase I investigation in 2023, no update has been published.
PPH is fairly well managed in high-resource settings, thanks to the availability of medicines and skilled medical care, but is a major cause of mortality in low-resource settings. R&D should focus on LMIC-applicable solutions, but only three of the marketed medicines and three of the candidates in development do not require cold-chain transportation and storage. Targeted efforts with low-resource settings limitations in mind are still needed to improve outcomes. In the meantime, low-cost devices represent a life-saving alternative.
In total, 28 devices were identified as in use or in active clinical development for postpartum haemorrhage. Of these, exactly half are already approved products (14) and half are in development (14).
Of the 14 approved devices, the largest portion are uterine balloon tamponade devices (UBTs) (six, 43%), followed by vacuum/suction devices (two devices, 14%), external compressive devices (two) and gauzes (two). There is also one intravaginal clamp and an arterial occlusion balloon. Nine approved products (64%) were designed and developed specifically to treat and control PPH, including several UBTs such as the Bakri Balloon and Ellavi Uterine Balloon Tamponade and the two vacuum/suction devices, the Jada System and the Barbyl Safe Labor Device. There are just five approved products (36%) which were repurposed, including celox and non-medicated gauzes, as well as non-pneumatic and pneumatic anti-shock garments.
In contrast to the approved products, a larger proportion of candidate devices under investigation are repurposed (5 devices, 36%) or improvised (3 devices, 21%), with the latter including the condom catheter, glove tamponade and oxytocin impregnated gauze. Just six devices specifically designed to treat PPH were studied in the past three years, including the PPH butterfly, which relies on bimanual compression and the Alma System, another vacuum/suction device. There are only three UBTs under investigation, including the Foley catheter, condom catheter and glove tamponade – all of which are used off-label already to treat postpartum haemorrhage. The other investigational devices are more varied in their approaches: there are five vacuum/suction devices (36%), one gauze, one intravaginal clamp and one external compressive device, comprising the ExAC fist protector, which is designed to facilitate manual external aortic compression. Other devices include the PPH butterfly, DAISY uterine drain and rudimentary ice packs placed around the abdomen to reduce blood loss. All devices under investigation are in human safety and efficacy trials, with none in discovery or preclinical. A lack of early-stage candidates might reflect a lack of dynamism, with a focus on trialling low-tech existing devices for use in PPH over the development of entirely novel approaches. While pragmatic, without new device concepts, the pipeline replenishment in the coming years could stall.
Postpartum haemorrhage pipeline
With a known fact that PPH remains the single biggest killer of new mothers, yet global R&D devoted to solving it fell to just US$ 8.6 million last year, barely a drop compared with the 70,000 lives lost annually and the millions more left with life-long complications. This mismatch means only a handful of heat-stable uterotonics and low-tech devices have reached the market, and the pipeline behind them is perilously thin and dominated by repurposed products rather than true innovation. To close this gap, funders, including industry and development partners, should deploy multi-year capital to underwrite late-stage trials, rigorous evidence generation and market-shaping efforts that bring affordable, LMIC-ready medicines and devices to scale. At the same time, catalytic grants must seed early-stage research into novel heat-stable uterotonics and simplified delivery technologies that remain effective without cold-chain logistics, making them viable in the very settings where most PPH deaths occur. Investing now is not only a moral imperative; economic analyses show that well-chosen PPH interventions are highly cost-effective and could avert tens of thousands of preventable maternal deaths every year. Resourcing the entire pipeline, from laboratory bench to last-mile clinic, is the fastest way to ignite the first genuine wave of innovation in PPH care in more than a generation.